Fig. 6.
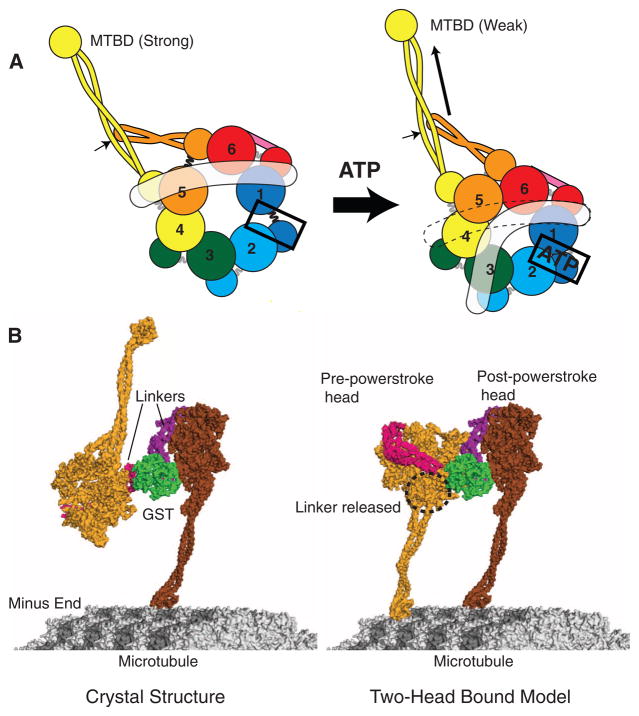
Models for dynein conformational changes. (A) A schematic model showing how ATP binding to AAA1 might propagate a conformation change to the MTBD and linker (in white). (Left) The apo state with gaps between AAA1 and 2 and between AAA5 and 6, as based on our crystal structure (Fig. 3B). (Right) The proposed consequence of ATP binding to AAA1: closure of the AAA1-2 gap, which in turn pulls upon and moves AAA2, 3, and 4. The relative motions that might ensue between AAA4 and 5 cause the detachment of the linker from AAA5. The indicated position of the linker in the ATP state is based on EM studies of Roberts et al. (8), although it may be mobile and not have a defined docked state on the ring. AAA4 and 5 movement may create a shear between the stalk (yellow coiled coil) and buttress (orange coiled coil); if the stalk preferentially interacts with the buttress through one of its helices, this could shift the registry of the stalk helices (arrow) that propagates to the MTBD to change its affinity (18, 34). (B) (Left) The dynein crystal structure with the distal stalk and MTBD, modeled as in Fig. 5A. One of the MTBDs is docked onto the microtubule as determined previously (18). (Right) One possible model in which the second head (light orange) has its MTBD docked onto the microtubule. To allow this head and its MTBD to bind to the microtubule, its linker was undocked from AAA5 and rotated about subdomain 3, and a slight bend was applied to the stalk of the front (left) head.