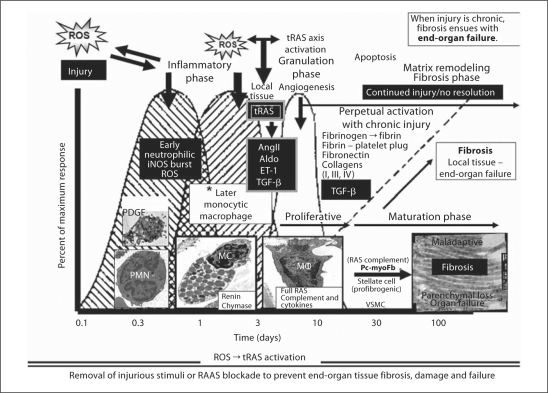
Fig. 4.
The innate local tissue wound healing response to injury. There is a commonality of organ-tissue injury to cutaneous wound repair [33]. The intended goal of the innate local tissue wound repair and healing response to injury is the return to wholeness or recovery – ‘restitutio ad integrum’. The generation of ROS as a result of metabolic, hormonal, and inflammatory injury, hypoxia, and recurrent trauma mechanisms in the CRS and T2DM is the injury responsible for the activation of the local tissue wound healing response. The injurious process is chronic as it is in the CRS and T2DM; however, the wound healing process does not result in recovery or a return to wholeness. Instead, it results in excessive ECM accumulation with fibrosis and scarring. The sequential and overlapping processes of hemostasis (platelets: platelet-derived growth factor, PDGF, and innate clotting factors), inflammation (acute and chronic, including activation and degranulation of resident MC), proliferation/granulation phase, ECM maturation/remodeling maturation and resolution/recovery are lost if the wound healing response is chronic and ongoing due to continued injury by ROS and strongly implicates a ROS-tRAS axis. Currently, the authors feel that the tRAS axis is activated in and around the inflammatory phase/granulation-proliferative and maturation phase nexus. Activated resident MC, chronic inflammatory cells, and fibrogenic cells along with tRAS activation, AngII generation along with aldosterone (Aldo) and ET-1, and TGF-β result in excessive ECM accumulation and fibrosis if tRAS is chronically activated. Importantly, MC are known to contribute renin and chymase from their secretory granules, the macrophage contains a full RAS complement, and the profibrotic myofibroblasts/pericytes or stellate cells contain a full RAS complement contributing to the maladaptive ECM remodeling fibrosis resulting in organ dysfunction and failure. Insets depict platelets, polymorphonuclear neutrophils (PMN), acute inflammatory cells (MC), chronic inflammatory cells [macrophages (MΦ)], and maladaptive ECM accumulation of collagen with end-organ fibrosis, dysfunction, and eventual failure.