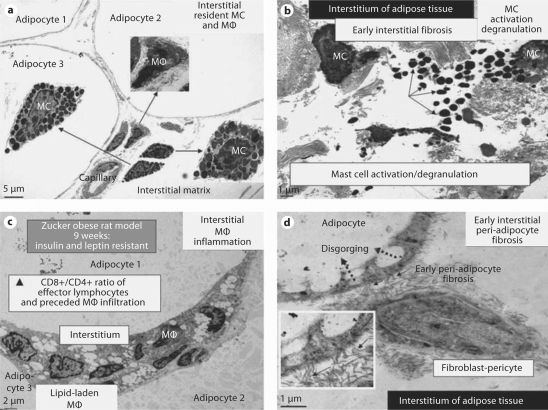
Fig. 5.
MC activation and degranulation with associated inflammation and fibrosis in adipose tissue of Zucker obese (fa/fa) rats at 9 weeks of age. The presence of MC in the interstitium of the obese subcutaneous and visceral adipose tissue in the young Zucker (fa/fa) model of obesity and insulin resistance with CRS and impaired glucose tolerance at 9 weeks of age (a × 300; bar = 5 μm) with associated inflammation and fibrosis. None of these observations was noted in the Zucker lean littermates. b MC activation and degranulation. Note the numerous liberated highly-electron-dense free MC secretory granules in the interstitium (arrows). ×1,000; bar = 1 μm. c The ensuing chronic inflammation with numerous lipid-laden macrophages (MΦ) in the interstitium of the omental adipose tissue. × 500; bar = 2 μm. d The ensuing early peri-adipocyte fibrosis (solid arrows) that progresses with continued chronic injury and the wound healing response. Note the disgorging of cytoplasmic lipid vacuoles into the large lipid-laden granule of the adipocyte (dotted arrows). Exploded inset depicts a higher magnification of collagen adhering to the adipocyte (arrows). There also exists pericapillary fibrosis (not shown). × 3,000; bar = 1 μm. In the past, we have thought classically that the fibroblast was important for fibrosis, however we are constantly learning that the pericyte is also capable of synthesizing collagen and that pericytes can differentiate into fibroblast-myofibroblast cell types. We currently speculate that this direct adherence (direct cell-matrix interaction) of collagen to the adipocyte (peri-adipocyte fibrosis) may result in a relative inability of adipocytes to undergo delipidation.