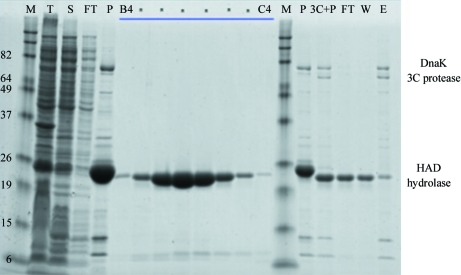
Figure 2.
A GelCode Blue-stained (Thermo Scientific, Rockford, Illinois, USA) SDS–PAGE of samples from a typical purification, represented in this case by recombinant HAD-superfamily hydrolase from Ehrlichia chaffeensis. Lanes are labelled as follows: M, molecular-weight standards; T, total protein; S, soluble fraction; FT, flowthrough (nonbound) from the first IMAC column; P, purified protein after first IMAC column; B4–C4, successive size-exclusion chromatography (SEC) fractions from peak (see Fig. 2 ▶), the dotted fractions were pooled for final concentration; 3C+P, protein after overnight cleavage with 3C protease; FT, unbound protein from second IMAC column after dialysis with 3C protease; W, protein from second IMAC column that eluted in the wash fractions; E, protein eluted from the second IMAC column with 500 mM imidazole. The identity of the DnaK protein band was determined by gel extraction, trypsin digest and mass-spectrometric analysis.