Figure 9.
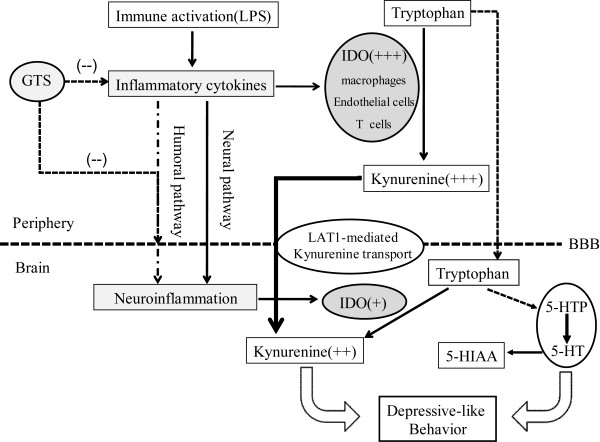
Proposed anti-depression mechanisms of GTS in the LPS-induced depression model. Peripheral LPS administration results in production of cytokines, including IL-1β, IL-6, IFN-γ), and TNF-α. These peripheral inflammation signals may be transmitted to the brain through humoral and neural pathways, leading to neuroinflammation. The peripheral and brain cytokines induce transcription and enzymatic activity of IDO, and lead to high levels of kynurenine in periphery and brain. As reported previously, peripheral kynurenine is the main source of increased kynurenine levels in LPS-treated mouse brain through LAT-1-mediated kynurenine active transport across the BBB. Increased kynurenine may metabolize to neurotoxic metabolites like quinolinic acid, thus influencing glutamatergic neurotransmission. Increased IDO activity may also decrease tryptophan availability, thus impacting serotonergic neurotransmission. Inflammation-associated disorder of serotonergic and glutamatergic neurotransmission ultimately induces depression-like behavior. GTS, a well known tonic traditional medicine with poor brain distribution, can improve depression-like behavior in LPS-challenged mice. This seemingly paradoxic concentration-effect relationship could be partially explained by an indirect periphery-to-brain pathway: GTS inhibits LPS-induced peripheral inflammation, thus decreasing neuroinflammation. The inhibition of peripheral inflammation could also partially reverse activation of IDO in the periphery, thus decreasing levels of kynurenine in brain which derive exclusively from periphery in this model, thus ameliorating depressive symptoms.