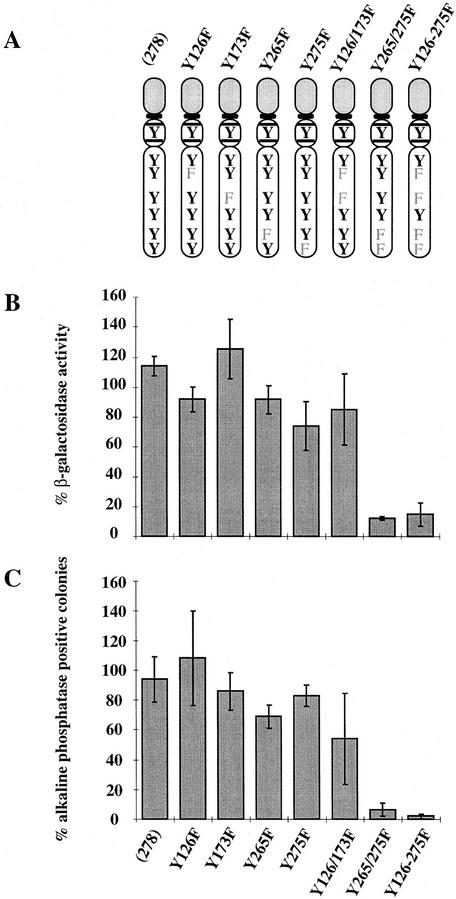
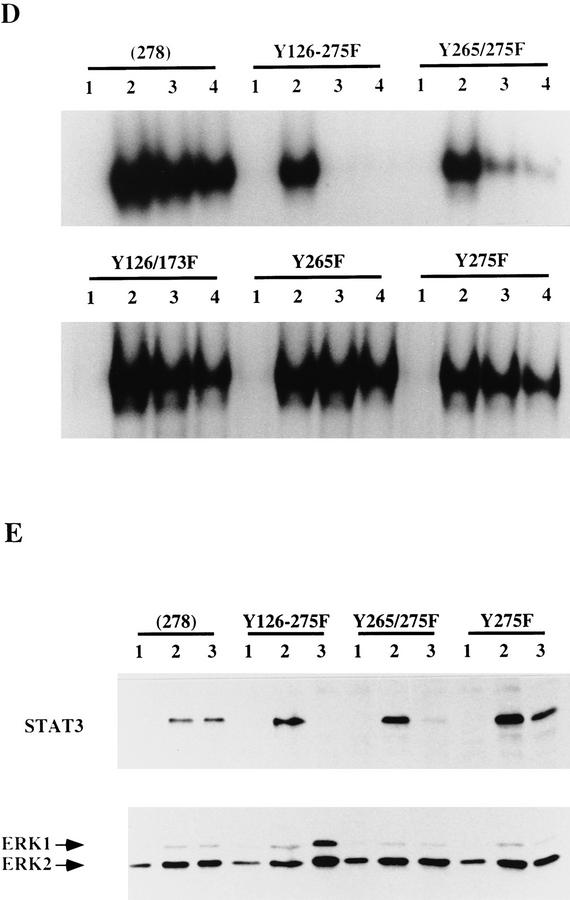
Figure 2.
Effect of mutating STAT3 interaction sites in gp130 on ES cell self-renewal and induction of STAT3 DNA-binding activity. (A) Schematic of the various chimeric receptors indicating the tyrosine–phenylalanine substitutions introduced into the wild-type (278) gp130 cytoplasmic domain. Numbering commences with the first residue of the 278-amino-acid intracellular domain of mouse gp130. The phenylalanine (F) for tyrosine (Y) substitutions in the four STAT3 docking sites are indicated. The additional three tyrosines do not interact with STAT3 (Stahl et al. 1995). (B) Stem cell renewal mediated by chimeric receptors in response to G-CSF measured by β-galactosidase expression from the Oct-4 locus. Data are mean ± s.e.m. for duplicate determinations on three independent clones normalized relative to response to IL-6/sIL-6R. (C) Efficiency of clonal stem cell renewal mediated by chimeric receptors in response to G-CSF measured by formation of alkaline phosphatase positive colonies. Data are mean ± s.e.m. for duplicate assays on three independent clones normalized relative to response to IL-6/sIL-6R. (D) Electrophoretic mobility-shift assay of induced STAT3 DNA binding. Transfected clones were left untreated (lane 1) or stimulated for 30 min with IL-6/sIL-6R (lane 2) or with G-CSF at 30 ng/ml (lane 3) or 3 ng/ml (lane 4). Nuclear extracts were assayed for SIE binding. (E) Immunoblot of STAT3 and ERK phosphorylation induced by G-CSF stimulation of chimeric receptors. Transfected clones were left untreated (lane 1) or were stimulated for 20 min with IL-6/sIL-6R (lane 2) or with G-CSF (lane 3). Immunoblots of cell lysates were probed sequentially with antibodies specific for the active phosphorylated forms of ERK and STAT3.