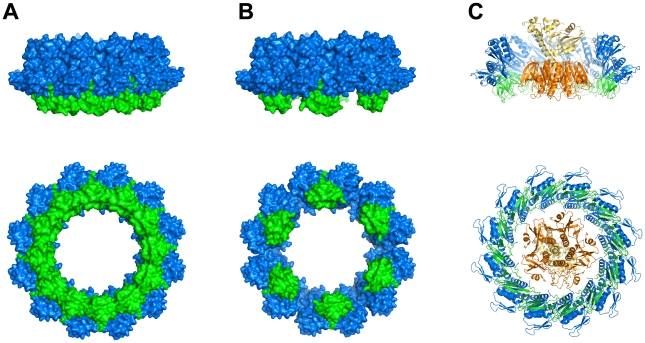
Figure 7. Combining structural data of GspD and GspC and the exoprotein cholera toxin.
(A) Two perpendicular views of the dodecameric ring of GspDN0-N1 (blue) obtained from crystallographic and electron microscopy studies [20], [24] with a dodecameric ring of GspCHR (green) assembled as described in the text. (B) Two perpendicular views of the same dodecameric ring of GspDN0-N1 (blue) shown in (A) with six GspCHR subunits (green) binding to alternating GspD subunits as described in the text. (C) Two perpendicular views of the exoprotein cholera toxin (B pentamer in gold, A subunit in yellow) positioned inside the dodecameric GspCHR–GspDN0-N1 ring depicted in (A). The five-fold axis of the B-pentamer is aligned by hand with the twelve-fold axis of the ring. The orientation of the AB5 hexamer with respect to the dodecamer is otherwise arbitrary. The cross-section of the double dodecamer of GspDN0-N1 and GspCHR is just sufficient to permit binding of the toxin heterohexamer. Obviously, the alternative assembly shown in (B) would also provide sufficient space for the toxin to bind at this location.