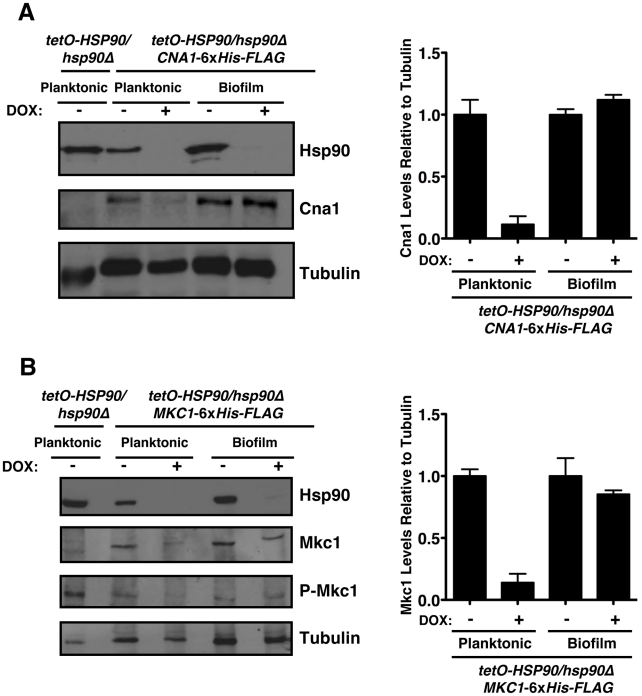
Figure 6. The Hsp90 client proteins Cna1 and Mkc1 exhibit reduced dependence on Hsp90 for stability under biofilm compared to planktonic conditions.
(A) Genetic depletion of Hsp90 does not reduce calcineurin levels in biofilm conditions. The tetO-HSP90/hsp90Δ strain with one allele of CNA1 C-terminally 6xHis-FLAG tagged was grown in planktonic or biofilm conditions with or without doxycycline (DOX, 20 µg/mL) for 48 hours. Total protein was resolved by SDS-PAGE and blots were hybridized with α-Hsp90, α-FLAG to monitor calcineurin levels, and α-tubulin as a loading control (left panel). Cna1 levels from two independent Western blots were quantified using ImageJ software (http://rsb.info.nih.gov/ij/index.html). The density of bands obtained for Cna1 was normalized relative to the density of bands for the corresponding tubulin loading control. Levels were subsequently normalized to the untreated control for the planktonic or biofilm state (right panel). (B) Depletion of Hsp90 does not deplete Mkc1 in biofilm conditions. The tetO-HSP90/hsp90Δ strain with one allele of MKC1 C-terminally 6xHis-FLAG tagged was grown in planktonic or biofilm conditions with or without DOX for 48 hours. Total protein was resolved by SDS-PAGE and blots were hybridized with α-Hsp90, α-His6 to monitor Mkc1 levels, α-phospho-p44/42 to monitor dually phosphorylated Mkc1, and α-tubulin as a loading control (left panel). Mkc1 levels from two independent Western blots were quantified using ImageJ software. The density of bands for Mkc1 was normalized relative to the density of bands for the tubulin loading control. Levels were subsequently normalized to the untreated control for the planktonic or biofilm state (right panel).