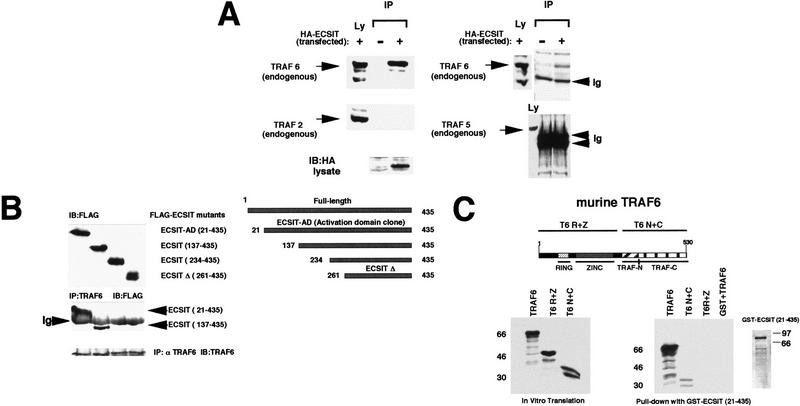
Figure 2.
ECSIT specifically interacts with TRAF6. (A) Coprecipitation of endogenous TRAF6 with transfected ECSIT. Lysates were prepared from untransfected and HA-tagged ECSIT-transfected 293 cells (left) and immunoprecipitated overnight with 20 μl of anti-HA monoclonal antibody and 25 μl of protein G–Sepharose. Sepharose beads were centrifuged and washed extensively, and bound endogenous protein was removed from the beads by addition of SDS-containing running buffer and boiling. Samples were divided in two, separated by SDS-PAGE, and immunoblotted with either an antibody to TRAF6 or an antibody to TRAF2. Lysates (Ly) that had not been immunoprecipitated were also assayed for the presence of transfected ECSIT (HA antibody) or for the presence of endogenous TRAF2 or TRAF6. COS cells (right) were assayed similarly for the ability of HA–ECSIT to immunoprecipitate TRAF5 (antibody from Santa Cruz). (IP) Immunoprecipitation; (Ly) lysates; (Ig) immunoglobulin. (B) In vivo mapping of the TRAF6 binding site on ECSIT. 293 cells were transfected with amino-terminal Flag epitope-tagged deletion constructs of ECSIT (schematic shown at right) and immunoprecipitated (IP) with TRAF6 antibody. Unprecipitated lysates were separated by SDS-PAGE (top) and assayed by immunoblotting (IB) with an antibody to Flag (M5). Immunoprecipitates were washed, denatured with SDS running buffer, boiled, and analyzed by SDS-PAGE (bottom). Membrane was blotted in Flag (M5) antibody to reveal interacting proteins. As a control the immunoprecipitated blot was reprobed with an antibody to TRAF6. (Ig) Immunoglobulin; (ECSIT–AD) ECSIT 21–435 originally cloned from yeast two-hybrid library; (ECSITΔ) ECSIT 261–435 deletion mutant. (C) In vitro mapping of the ECSIT binding site on TRAF6. A GST fusion protein of ECSIT (21–435) was prepared in bacteria and incubated with 35S-labeled in vitro translation products of TRAF6 including full-length TRAF6, the amino-terminal ring and zinc fingers of TRAF6 (T6 R+Z), and the carboxy-terminal TRAF domain (T6 N+C). Proteins binding to GST–ECSIT (21–435) were bound to glutathione–Sepharose beads, washed, and analyzed by PAGE (right). Two micrograms of GST alone was also incubated with full-length TRAF6 (GST+TRAF6) as a control.