Figure 7.
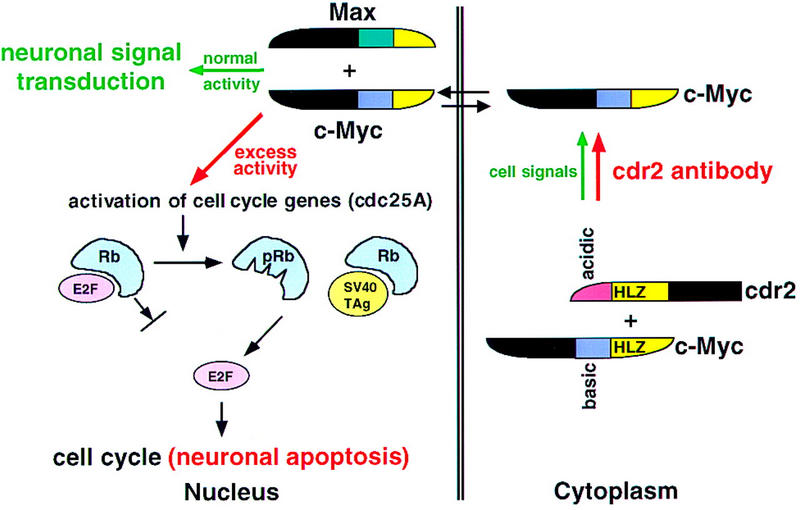
A model for the role of cdr2 antibodies in PCD pathogenesis. Cdr2 and c-Myc form a complex in Purkinje cell cytoplasm. It is presumed that this interaction is normally regulated by signals that allow c-Myc entry into the nucleus, where it acts to promote transcription and transduce neuronal signaling. In PCD, cdr2 antibodies are proposed to disrupt normal regulation of the cdr2:c-Myc interaction, leading to unregulated entry of c-Myc into the nucleus and aberrant c-Myc-induced gene transcription. c-Myc can drive cell cycle pathways or induce apoptosis in dividing cells (Evan and Littlewood 1998). For example, c-Myc activates cdc25A (Galaktionov et al. 1996), and cdc25 family members act on a number of downstream proteins leading to the phosphorylation of the retinoblastoma gene product (pRb) and the release of the transcription factor E2F, a common final step in S–phase entry. c-Myc also can induce ARF (Zindy et al. 1998), a protein that induces p53-mediated apoptosis (Prives 1998). Excess nuclear c-Myc in neurons is proposed to induce inappropriate cell cycle signaling and Purkinje cell apoptosis in a manner analogous to that observed in Purkinje promoter SV40 TAg transgenic mice (Feddersen et al. 1992, 1995). In these mice, T antigen binds Rb, leading to the release of E2F, S–phase entry, and Purkinje apoptosis. We suggest that cdr2 antibody-mediated disruption of the cdr2:c-Myc interaction leads to aberrant nuclear c-Myc activity, activation of these cell cycle and apoptotic pathways, and contributes to the pathogenesis of PCD.
