Abstract
The interaction between puerarin and β-cyclodextrin (CD) has been studied in D2O, H2O/acetone-d6, acetone-d6 and DMSO-d6 solutions by 1H NMR spectroscopy. The NMR data obtained from hydroxy protons indicate that the formation of the inclusion complex between the two molecules is not stabilized by strong hydrogen bond interactions. The sugar part of puerarin as well as the A ring are outside the β-CD cavity while the B and C rings are located inside the cavity and the interaction is mainly stabilized by hydrophobic interactions. In DMSO at 30°C and in acetone-d6/H2O at temperature below −5°C, doubling of some signals indicated that, in these solvent systems, free rotation of the C-glycosyl bond was restricted due to the steric hindrance between the phenolic hydroxy group at C-7 and the bulky sugar moiety at C-8. In acetone, fast exchange of phenolic protons on the NMR timescale was observed, showing the effect of the solvent on the hindered rotation.
Keywords: β-cyclodextrin, Hydroxy protons, Interaction, NMR, Puerarin
Introduction
Traditional Chinese medicines have been extensively used to prevent and cure many diseases in China owing to low toxicity and rare complications. Puerarin (7,4-dihydroxyisoflavone-8-glucopyranoside) is one of the major isoflavonoid compounds isolated from the root of Pueraria lobata (Willd.) Ohwi. It exhibits a great variety of biological and pharmacological activities such as antihypertension, anti-arteriosclerosis, dilating coronary arteries, decreasing myocardial oxygen consumption, and improving microcirculation in both animals and humans suffering from cardiovascular disease [1–5]. Due to these many biological activities, it is of importance to identify selective chromatographic methods that are able to purify puerarin from crude extracts.
β-cyclodextrin (β-CD) is a cyclic oligosaccharide [6–9] having a truncated cone structure with a hydrophilic external surface and a hydrophobic central cavity. This unique structure allows the formation of inclusion complexes with a large variety of molecules and to discriminate between various types of guest molecules by selectively incorporating such molecules through size and polarity considerations [10, 11]. Thus, CDs are widely used as drug carrier systems [6], in separation technology [12–14] and other areas [15, 16]. Several driving forces have been proposed for the inclusion of β-CD with guest molecules, such as hydrogen bonding, hydrophobic interactions and the release of high energy water molecules from the cavity. Recently, the use of β-CD-immobilized materials for puerarin separation has been investigated [17–19]. Investigating the interaction between β-CD and puerarin is of significance for the design of chromatographic media and the improvement of separating efficiency. Several different methods are used for the study of the guest–host interactions, including nuclear magnetic resonance (NMR) spectroscopy, calorimetry, capillary electrophoresis and potentiometry [20–24]. Among these methods, NMR is widely used, since several NMR parameters such as proton chemical shifts, nuclear Overhauser effects and diffusion coefficients will be affected in a characteristic manner upon interaction.
We have recently studied the formation of an inclusion complex between puerarin and β-CD using proton NMR chemical shifts and ROESY experiments [25] and the existence of a hydrogen bonding interaction was proposed from a molecular dynamic study [26]. However, no direct experimental proofs are yet available to demonstrate hydrogen bonding interaction between puerarin and β-CD. Thus, the aim of the present study was to characterize the inclusion complex formed between puerarin and β-CD using NMR spectroscopy of hydroxy protons to investigate if hydrogen bonding interactions between β-CD and puerarin participate in the stabilization of the complex.
Experimental
Materials Puerarin was purchased from the National Institute for the Control of Pharmaceutical and Biological Products, China, and β-cyclodextrin from Sigma–Aldrich Inc., China.
Sample preparation The samples of CD complexes were prepared to have 7.5 mM concentrations and a 1:1 molar ratio for both host and guest molecule. NMR spectra of β-CD and of puerarin alone were recorded at the same concentrations as those used to study the complexes.For determination of stoichiometry, equimolar amounts of β-CD and puerarin were dissolved in D2O. These solutions were distributed among nine NMR tubes, with the molar fraction of CD increasing from 0.1 to 0.9 in the resulting solutions, together with the molar fraction of puerarin decreasing from 0.9 to 0.1, at a constant total concentration of 7.5 mM.
NMR All NMR experiments were performed on a Bruker DRX 400 spectrometer using a 5-mm 1H/13C/15N/31P QNP probe equipped with z-gradient. For experiments in D2O, the chemical shifts were referenced relative to the DOH signal at δH 4.70 ppm. Two-dimensional COSY, TOCSY and ROESY NMR spectra were acquired using standard pulse sequences from the Bruker library. A spectral width of 3,188 Hz with 2 K data points in t2 and 256 in t1 were used. The relaxation delay between successive pulse cycles was 1.5 s for COSY and TOCSY and 2 s for ROESY. Mixing times of 80 and 200 ms were used for TOCSY and ROESY, respectively.
Experiments in 15% (CD3)2CO/85% H2O To minimize the absorption of impurities from the glassware, the NMR tubes were soaked for 24 h in 50 mM sodium phosphate buffer at pH 7 [27]. One- and two-dimensional 1H NMR spectra were acquired using the WATERGATE pulse sequence [28] for water suppression, and the calibration of proton chemical shifts was done by setting the residual acetone-d5 signal to δH 2.204 ppm. The temperature coefficients (dδ/dT) for the hydroxy protons were obtained from 1H NMR spectra recorded between −8°C and 10°C in 5°C steps.
DOSY experiments Data acquisition and analysis were performed using the Bruker TOPSPIN software (version 1.3). The DOSY experiments were performed at 30°C using the ledbpgp2s pulse sequence from the Bruker library, with stimulated echo, longitudinal eddy current compensation, bipolar gradient pulses, and two spoil gradients using 16 different gradient values varying from 2% to 95% of the maximum gradient strength. One hundred milliseconds diffusion time was chosen for samples in D2O. The gradient length was set to 2.2 ms. Processing was achieved using 4096 points in the F2 dimension and 256 points in F1. An exponential window function with 1 Hz line broadening was applied in the F2 dimension prior to Fourier transformation. After baseline correction, the diffusion dimension was processed with the DOSY processing program (Bruker TOPSPIN software 2.0). A logarithmic scaling was applied along the diffusion axis, and a noise sensitivity factor of 2 and line width factor of 1 were used. The fitting of the diffusion dimension in the 2D-DOSY spectra was obtained using a single exponential fit (Nexp = 1). The DOSY experiment was carried out twice and for two different sample preparations.
Results
Stoichiometry of the complex
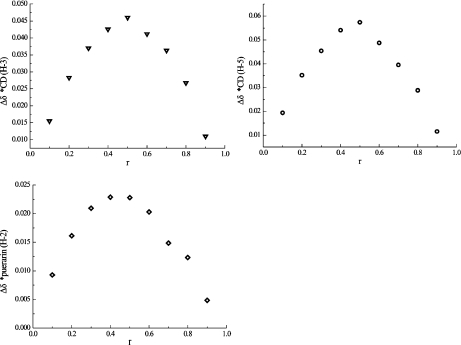
The stoichiometry of the complex was determined using the continuous variation method (Job’s method) [29]. The complexation-induced shifts of H-3 and H-5 of β-CD and H-2 of puerarin are shown on the Job plots in Fig. 1, where the apex at 0.5 indicates the principal formation of a 1:1 complex between puerarin and β-CD.
Fig. 1.
Job plots. r is the molar fraction of β-CD in the puerarin/β-CD mixture
Complexation in D2O
Only one set of NMR signals was observed for the complex between puerarin and β-CD (1:1) indicating a rapid exchange between free and bound forms on the NMR timescale.
Chemical shifts The addition of puerarin to a β-CD solution resulted in the shielding of H-3 and H-5 positioned on the inner surface of β-CD, as well as of H-6 located on the cavity rim at the narrow end of the molecule (Table 1). Even H-1, H-2 and H-4 located on the outer surface of the torus experienced a small upfield shift. The shielding is due to the ring current effect of the aromatic systems in puerarin and indicates that puerarin is entering the CD cavity. The upfield shift of the H-5 proton is the most prominent (−0.11 ppm) followed by H-3 (−0.09 ppm) and by H-6 (0.06 ppm). Table 2 shows that the protons on puerarin were also affected by the addition of β-CD. In the presence of β-CD, H-2 and H-2′,6′ were shielded, while H-5 and H-6 were deshielded, suggesting that the B and C rings of puerarin entered into the cavity of β-CD.
Table 1.
1H NMR chemical shifts (δ, ppm) for CH protons of β-CD alone, and their complexation-induced shifts (CIS) when in complex with puerarin (30°C, D2O)
| H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | |
|---|---|---|---|---|---|---|
| δ | 5.042 | 3.621 | 3.935 | 3.555 | 3.824 | 3.849 |
| CIS | − 0.024 | − 0.025 | − 0.095 | − 0.011 | − 0.107 | − 0.057 |
Table 2.
1H NMR chemical shifts (δ, ppm) for CH protons of puerarin alone and their complexation-induced shifts (CIS) when in complex with β-CDs (30°C, D2O)
| D2O | H-2 | H-5 | H-6 | H-2′6′ | H-3′5′ | H-1″ | H-2″ | H-3″ | H-4″ | H-5″ | H-6″ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| δ | 8.209 | 8.061 | 7.085 | 7.364 | 6.957 | 5.145 | 4.264 | 3.621 | 3.621 | 3.621 | 3.894 |
| 3.793 | |||||||||||
| CIS | − 0.069 | 0.079 | 0.030 | − 0.043 | − 0.008 | − 0.005 | 0.018 | a | a | a | 0.015 |
a not determined due to spectral overlap
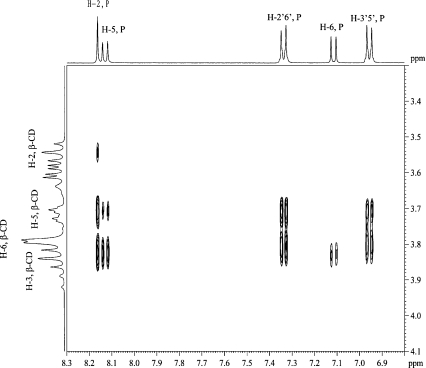
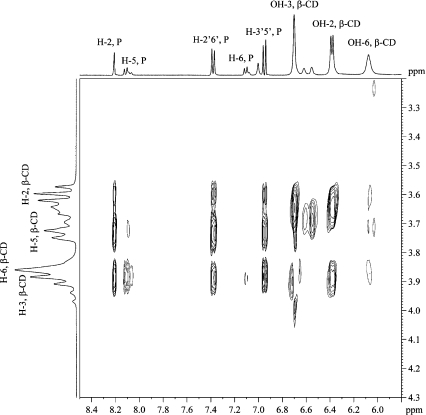
ROESY The 2D ROESY spectrum of the complex (Fig. 2) showed cross-peaks corresponding to strong ROEs between H-2, H-2′,6′ and H-3′,5′ of puerarin and H-5 and H-3 of β-CD. Medium ROEs are found between H-5 of puerarin and H-3 of β-CD, while weak ROEs exist between H-5 of puerarin and H-5 of β-CD as well as between H-6 of puerarin and H-3 of β-CD. No cross-peak was found between H-6 of puerarin and H-5 of β-CD. These ROEs show that the B and C rings of puerarin are in the cavity of β-CDs and indicate that puerarin penetrates the β-CD cavity from the wide rim.
Fig. 2.
Part of the ROESY spectrum of the 1:1 β-CD/puerarin complex in D2O, 200 ms, 30°C
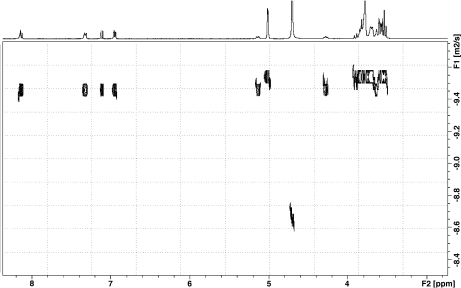
DOSY DOSY unequivocally confirmed the formation of a stable complex between β-CD and puerarin. The diffusion coefficients of free puerarin and β-CD are 4.38 and 2.87 · 10 − 10 m2 s − 1, respectively. In presence of β-CD, puerarin showed a decrease in diffusion rate with a diffusion coefficient of 3.39 · 10 − 10 m2 s − 1, indicating the formation of a complex (Fig. 3). The reference HDO diffusion coefficient of 2.15 · 10 − 9 m2 s − 1 was in good agreement with published data [30]. Taking into account that the studied system is under fast equilibrium on the NMR timescale, the observed diffusion coefficient is the weighted average of those of the free and bound molecules, due to the fast exchange of free and bound species on the NMR timescale:
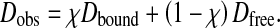 |
1 |
Dobs is the observed diffusion coefficients while Dfree and Dbound are the diffusion coefficients of free and bound puerarin, respectively. The association constant K was determined using Eq. 2, with the assumption of a known mole fraction χ of the bound guest χb:
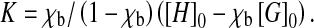 |
2 |
[H]0 and [G]0 are the total concentration of the host and guest, respectively. An association constant K of 2,000 ± 500 M − 1 was obtained using single-point procedure [31, 32] in which it is assumed that the diffusion coefficient of the host–guest complex is the same as that of the host molecule. The K value should only be taken as an indication of the relative affinity of the puerarin for β-CD due to the large uncertainty introduced by the single-point approximation method. This method has, however, been shown to yield values for the β-CD/epicatechin and β-CD/epigallocatechin gallate complexes similar to those obtained with other methods [33].
Fig. 3.
DOSY spectrum of puerarin/β-CD (1:1) in D2O and at 30°C
Complexation in 85% H2O/15% (CD)3CO
To investigate if the complex formed between β-CD and puerarin was stabilized by hydrophilic interactions such as hydrogen bonds [34], the exchangeable hydroxy protons have been also studied by 1H NMR (Fig. 4). To be able to observe hydroxy protons by NMR, their rate of exchange with water has to be reduced. To achieve this, hydroxy protons are usually studied at low temperature in a solvent mixture of H2O and acetone-d6 [35].
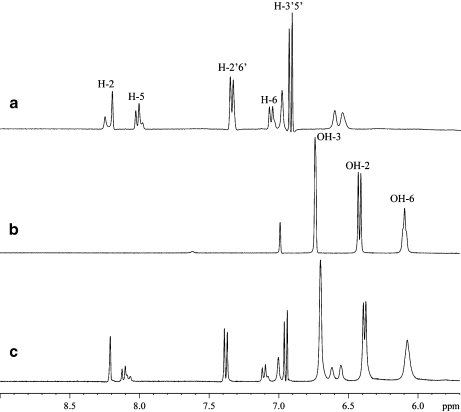
Fig. 4.
1H NMR spectra showing the region for aromatic and hydroxy proton signals of a 1:1 β-CD/puerarin complex in 85% H2O/15% acetone-d6 at −8°C; a puerarin alone; b β-CD alone; c 1:1 β-CD/puerarin complex
Thus, the chemical shifts (δ), temperature coefficients (dδ/dT), 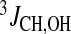 coupling constants and ROEs signals between β-CD and puerarin were measured to monitor hydration and hydrogen bonding interactions. Protons involved in hydrogen bonding are usually deshielded; however, in strongly hydrated systems such as carbohydrates, we have shown that an upfield shift will indicate reduced hydration due to steric hindrance or hydrogen bonding with, for example, ring oxygens, whereas a downfield shift will show proximity to other hydroxyl groups [36]. The chemical shift of a hydroxy proton involved in a hydrogen bond or with reduced hydration is less affected by temperature changes due to decreased interaction with the solvent. Thus, hydroxy protons with large temperature coefficients ∣ dδ/dT ∣ > 11 ppb/°C are fully hydrated, whereas hydroxy protons with ∣ dδ/dT ∣ < 11 ppb/°C are only partially hydrated. For strong hydrogen bonding or strong reduced hydration, ∣ dδ/dT ∣ values of less than 5 ppb/°C have been measured [35, 37, 38]. Coupling constants that do not represent conformational averaging (ca. 5 Hz) can also be indicative of hydrogen bond interaction.
coupling constants and ROEs signals between β-CD and puerarin were measured to monitor hydration and hydrogen bonding interactions. Protons involved in hydrogen bonding are usually deshielded; however, in strongly hydrated systems such as carbohydrates, we have shown that an upfield shift will indicate reduced hydration due to steric hindrance or hydrogen bonding with, for example, ring oxygens, whereas a downfield shift will show proximity to other hydroxyl groups [36]. The chemical shift of a hydroxy proton involved in a hydrogen bond or with reduced hydration is less affected by temperature changes due to decreased interaction with the solvent. Thus, hydroxy protons with large temperature coefficients ∣ dδ/dT ∣ > 11 ppb/°C are fully hydrated, whereas hydroxy protons with ∣ dδ/dT ∣ < 11 ppb/°C are only partially hydrated. For strong hydrogen bonding or strong reduced hydration, ∣ dδ/dT ∣ values of less than 5 ppb/°C have been measured [35, 37, 38]. Coupling constants that do not represent conformational averaging (ca. 5 Hz) can also be indicative of hydrogen bond interaction.
Hydroxy protons in β-CD and puerarin alone The assignment of the hydroxy proton signals in β-CD was obtained from DQF-COSY spectra. The chemical shifts, vicinal coupling constants and temperature coefficients (dδ/dT; Table 3) are in good agreement with previously reported values [34]. Although there are six hydroxy protons in puerarin, only four hydroxy proton signals were observed in the NMR spectra. These protons were assigned from DQF-COSY and TOCSY to the hydroxy protons of the sugar portion of the molecule (Table 4). No signals were observed for the hydroxy protons OH-7 and OH-4′ from the aromatic rings A and C. Attempts to observe these signals were made by changing the sample pH from 6.2 to 3.5 but were not successful. It should be noted that at temperature below −5°C, two sets of resonances were observed for H-2, H-5, and H-6 as well as for H-2′/H-6′ in ratio 0.56:1 (vide infra).
Table 3.
1H NMR Chemical shift (δ, ppm), CIS (ppm) and temperature coefficients (dδ/dT, ppb/°C) for OH of β-CD at -8°C in 85% H2O / 15% acetone-d6
| OH-2 | OH-3 | OH-6 | |
|---|---|---|---|
| δ | 6.424 | 6.742 | 6.097 |
| CIS | − 0.040 | − 0.041 | − 0.019 |
| JCH, OH | 7.4 | <3 | <3 |
| dδ/dT | − 12.5 | − 15.4 | − 14.2 |
| dδ/dT (Puerarin) | − 7.7 | − 9 | − 13.4 |
Table 4.
1H NMR Chemical shift (δ, ppm), CIS (ppm) and temperature coefficients (dδ/dT, ppb/°C) for hydroxy protons of puerarin at −8°C in 85% H2O / 15% acetone-d6
| OH-2″ | OH-3″ a | OH-4″ a | OH-6″ | |
|---|---|---|---|---|
| δ | 6.559 | 6.621 | 6.621 | 6.338 |
| CIS | 0.008 | 0.012 | 0.012 | 0.021 |
| dδ/dT | − 12.4 | − 13.6 | − 13.6 | b |
| dδ/dT (β-CD) | − 12.1 | − 13.7 | − 13.7 | b |
a the OH-3″ and OH-4″ signals were overlapping over the all range of temperature
b could not be determined
Hydroxy protons in β-CDs and puerarin in the complex Comparison of the chemical shifts of the hydroxy proton signals of β-CD alone and in the complex shows that upon formation of the complex, OH-2 and OH-3 are shielded by 0.040 and 0.041 ppm, respectively, while OH-6 is upfield shifted by 0.017 ppm. In β-CD alone, OH-2 and OH-3 had temperature coefficient of −12.5 and −15.4 ppb/°C, respectively, while in the complex, these values decreased to −7.7 and −9.0 ppb/°C. Thus, upon inclusion of puerarin, water is excluded from the CD cavity, and the hydration of OH-2 and OH-3 is reduced [39]. The smaller chemical shift change and the higher temperature coefficient of OH-6 indicate more contact with water. No changes in the 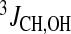 values of OH-2, OH-3 and OH-6 of β-CD were observed upon complex formation. As for puerarin alone, it was not possible to observe the hydroxy proton signals OH-7 and OH-4′ of the aromatic rings of puerarin in the complex. There were no major changes in the chemical shifts and temperature coefficients of the hydroxy protons of the sugar moiety of puerarin, supporting the NMR data obtained in D2O that showed that the sugar part of the molecule was not inside the CD cavity. The 2D ROESY spectra of the 1:1 complex in 85% H2O/15% (CD)3CO showed ROEs in good agreement with those observed in D2O solution (Fig. 5). This, together with similar chemical shifts of the CH protons in D2O and in 85% H2O/15% (CD)3CO solutions, indicate that the presence of acetone-d6 does not change the formation and the structure of the complex. No ROE involving hydroxy protons of β-CD or of the sugar part of puerarin was observed. At temperature below −5°C, two sets of resonances in a 1:0.5 ratio were observed for H-5 and H-6 on the A ring of puerarin, but H-2 from the B ring and H-2′/H-6′ from the C ring showed only one set of resonances if compared to puerarin alone.
values of OH-2, OH-3 and OH-6 of β-CD were observed upon complex formation. As for puerarin alone, it was not possible to observe the hydroxy proton signals OH-7 and OH-4′ of the aromatic rings of puerarin in the complex. There were no major changes in the chemical shifts and temperature coefficients of the hydroxy protons of the sugar moiety of puerarin, supporting the NMR data obtained in D2O that showed that the sugar part of the molecule was not inside the CD cavity. The 2D ROESY spectra of the 1:1 complex in 85% H2O/15% (CD)3CO showed ROEs in good agreement with those observed in D2O solution (Fig. 5). This, together with similar chemical shifts of the CH protons in D2O and in 85% H2O/15% (CD)3CO solutions, indicate that the presence of acetone-d6 does not change the formation and the structure of the complex. No ROE involving hydroxy protons of β-CD or of the sugar part of puerarin was observed. At temperature below −5°C, two sets of resonances in a 1:0.5 ratio were observed for H-5 and H-6 on the A ring of puerarin, but H-2 from the B ring and H-2′/H-6′ from the C ring showed only one set of resonances if compared to puerarin alone.
Fig. 5.
Part of the ROESY spectrum of the 1:1 β-CD/puerarin complex in 85% H2O/15% acetone-d6, 200 ms, −8°C
NMR study in acetone-d6 and DMSO-d6 solutions
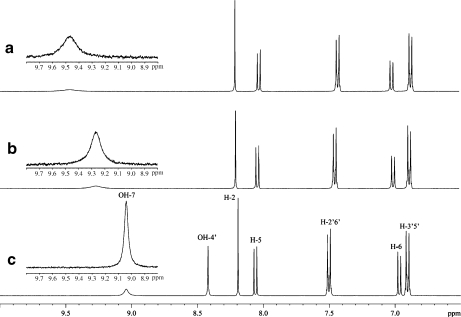
In an attempt to identify the hydroxy protons of the aromatic rings, NMR spectra of puerarin in acetone-d6 and DMSO-d6 solutions were also obtained. In acetone at 30°C, the two resonances at 9.041 and 8.421 ppm downfield from the CH aromatic signals of puerarin (Fig. 6) were assigned to the hydroxy protons OH-4′ and OH-7. In the ROESY spectrum (data not shown), ROEs was observed between the most downfield signal and H-6 and between the most upfield signal and H-3′/5′. OH-4′ appeared as a sharp signal while OH-7 was broad. The addition of 5% H2O resulted in the disappearance of the OH-4′ signal and in the broadening and downfield shift of OH-7 (Fig. 6) due to exchange with water and hydrogen bond interaction with water, respectively. After addition of 20% H2O, the OH-7 signal disappeared due to a rapid exchange process.
Fig. 6.
1H NMR spectra of puerarin in acetone and acetone/H2O solutions; a puerarin in 80% acetone-d6/20% H2O; b puerarin in 90% acetone-d6/10% H2O; c puerarin in 100% acetone-d6
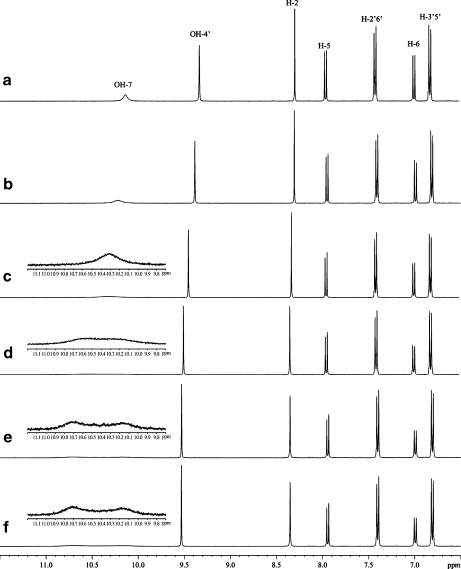
In the 1H NMR spectrum in DMSO-d6 at 25°C, the OH-4′ signal appeared as a sharp resonance as observed in acetone, while two sets of signals were seen for OH-7. The NMR spectra at various temperatures are shown in Fig. 7. These two signals in a 1:1 ratio reveal two conformational isomers created by rotational hindrance at the glucosyl-flavone linkage. The equilibrium between the two rotamers was supported by observations of strong exchange cross-peaks between the two signals in the ROESY spectrum. Coalescence of the two signals was observed upon increasing the temperature to 40°C. It should be noted that no changes in proton chemical shifts of puerarin and β-CD and no intermolecular ROEs between proton of puerarin and β-CD were observed, suggesting that complex formation dos not occur in DMSO solution (Fig. 8).
Fig. 7.
1H NMR spectra of puerarin in DMSO-d6 at different temperatures; a 60°C; b 50°C; c 40°C; d 30°C; e 25°C; f puerarin in the presence of β-CD at 25°C
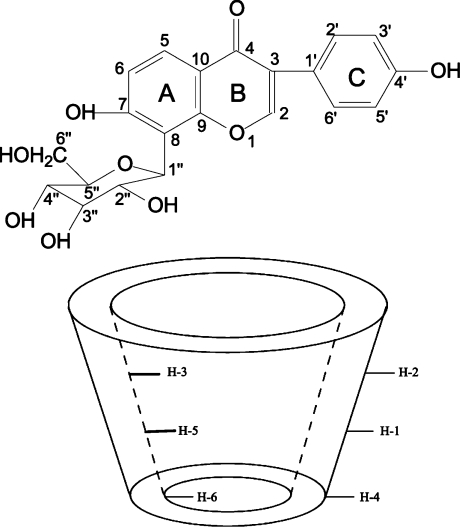
Fig. 8.
Structure of puerarin and β-CD
Discussion
MD simulation in water solvent [26] showed that two different types of inclusion complex of β-CD/puerarin could be formed, the difference in the two types of complexes being the orientation of puerarin inside the cavity of β-CD. In one complex, the C-ring of puerarin is inside the cavity of β-CD with the G unit close to the secondary rim, whereas, in the other type of complex, the G unit was closed to the primary ring. Hydrogen bonds were found to play an important role in bridging β-CD and puerarin together as well as in forming a stable complex. It has also been proposed that the retention mechanism between β-CD and puerarin involves not only hydrophobic interactions but also hydrogen bond interactions [40].
Our study shows that upon inclusion of puerarin, water is excluded from the CD cavity and the hydration of OH-2 and OH-3 of β-CD is reduced. Even if OH-7 and OH-4′ on the aromatic rings on puerarin could not be observed, the NMR data on the hydroxy protons of β-CD indicate that no strong hydrogen bond between the hydroxyl groups of β-CD and those of puerarin stabilize the structure of the complex. This is different from what we have previously observed in a study on the interaction between β-CD and epigallocatechin gallate, where the NMR data obtained from the hydroxy protons suggested that intermolecular hydrogen bonding in addition to hydrophobic interaction stabilized the complex [38].
The presence of rotamers leading to signal doubling has been observed for flavones containing an 8-C-hexosyl substituent [41–43]. Interaction between the flavone B-ring and the 8-C-hexosyl substituent leading to restricted rotation around the sugar C-aglycon bond has been suggested to give rise to a mixture of two NMR-distinguishable rotamers. Thus, the 8-C-monoglucosides of apigenin and luteonin exhibited signal duplication in their NMR spectra recorded in DMSO-d6 while the corresponding spectra of the 6-C-glucosides of apigenin and luteonin in which the C-glycosyl residues were relatively distant from the flavone B-rings did not show rotameric conformers. The present study shows also the effect of the solvent on the hindered rotation. Thus in acetone, the fast exchange of phenolic protons on the NMR timescale gives an average chemical shift spectrum. In DMSO at room temperature and in H2O/acetone solutions at low temperature, the exchange rate of the phenolic protons slows considerably, and signal doubling is observed for some protons.
Acknowledgements
The authors would like to thank Professor Lennart Kenne for inviting Rui Zhao to perform this study in his research group and Professor Jan-Christer Janson for valuable technical assistance. This research was financially supported by the National High Technology Research and Development Program of China (2006AA020203), the National Nature Science Foundation of China (20576013), the State Key Development Program for Basic Research of China (2007CB714304), the Natural Science Foundation of Beijing, China (2071002).
References
- 1.Song X, Chen P, Chai X. Effects of puerarin on blood pressure and plasma renin activity in spontaneously hypertensive rats. Acta Pharmacol. Sin. 1988;9:55–58. [PubMed] [Google Scholar]
- 2.Li Y, Yang Y. Clinical treatment by puerarin in patients with senile ischemic cerebrovascular disease. Chin. Pharm. J. 1997;32:776–777. [Google Scholar]
- 3.Liu Q, Lu Z, Wang L. Restrictive effect of puerarin on myocardial infarct area in dogs and its possible mechanism. J. Tongji Med. Univ. 2000;20:43–45. doi: 10.1007/BF02887673. [DOI] [PubMed] [Google Scholar]
- 4.Han J, Wang W, Wang L, Liu S, Kang T. Effect of puerarin and daidzein on proliferating vascular smooth muscle cells. Chin. J. Chin. Mater. Med. 2004;29:437–440. [PubMed] [Google Scholar]
- 5.Wu Z, Liu Y, Zhu Y, Yan H. Studies on lowering IOP effect of puerarin eyedrops. J. Chin. Pharm. Univ. 1998;29:387–389. [Google Scholar]
- 6.Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems. Chem. Rev. 1998;98:2045–2076. doi: 10.1021/cr970025p. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Purdy WC. Cyclodextrins and their applications in analytical chemistry. Chem. Rev. 1992;92:1457–1470. doi: 10.1021/cr00014a009. [DOI] [Google Scholar]
- 8.Saenger W, Jacob J, Gessler K, Steiner T, Hoffmann D, Sanbe H, Koizumi K, Smith SM, Takaha T. Structures of the common cyclodextrins and their larger analogues—beyond the doughnut. Chem. Rev. 1998;98:1787–1802. doi: 10.1021/cr9700181. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro ACF, Esteso MA, Lobo VMM, Valente AJM, Simoes SMN, Sobral AJFN, Ramos L, Burrows HD, Amado AM, Amorim da Costa AM. Interactions of copper (II) chloride with β-cyclodextrin in aqueous solutions. J. Carbohydr. Chem. 2006;25:173–185. doi: 10.1080/07328300600732469. [DOI] [Google Scholar]
- 10.Szejtli J. Cyclodextrins and Their Inclusion Complexes. Budapest: Akademiai Kiado; 1982. [Google Scholar]
- 11.Ali SM, Asmat F, Maheshwari A. NMR spectroscopy of inclusion complex of d-(-)-chloramphenicol with β-cyclodextrin in aqueous solution. Il Farmaco. 2004;59:835–838. doi: 10.1016/j.farmac.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong DW, Alak A, Bui K, DeMond W, Ward T, Riehl TE, Hinze WL. Facile separation of enantiomers, geometrical isomers, and routine compounds on stable cyclodextrin LC bonded phases. J. Incl. Phenom. 1984;2:533–545. doi: 10.1007/BF00662219. [DOI] [Google Scholar]
- 13.Smolková-Keulemansová E, Feltl L, Krýsl S. Chromatographic study of the inclusion properties of cyclodextrins: study of inclusion from the gaseous phase. J. Incl. Phenom. 1985;3:183–196. doi: 10.1007/BF00687992. [DOI] [Google Scholar]
- 14.Easton CJ, Lincoln SF. Chiral discrimination by modified cyclodextrins. Chem. Soc. Rev. 1996;25:163–170. doi: 10.1039/cs9962500163. [DOI] [Google Scholar]
- 15.Takahashi K. Organic reactions mediated by cyclodextrins. Chem. Rev. 1998;98:2013–2034. doi: 10.1021/cr9700235. [DOI] [PubMed] [Google Scholar]
- 16.Hedges A.R. Industrial applications of cyclodextrins. Chem. Rev. 1998;98:2035–2044. doi: 10.1021/cr970014w. [DOI] [PubMed] [Google Scholar]
- 17.He X, Tan T, Xu B, Janson J-C. Separation and purification of puerarin using β-cyclodextrin-coupled agarose gel media. J. Chromatogr. A. 2004;1022:77–82. doi: 10.1016/j.chroma.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 18.He X, Tan T, Janson J-C. Purification of the isoflavonoid puerarin by adsorption chromatography on cross-linked 12% agarose. J. Chromatogr. A. 2004;1057:95–100. doi: 10.1016/j.chroma.2004.09.068. [DOI] [PubMed] [Google Scholar]
- 19.Li R, Zhao R, Zhang H, Li C, Feng D, Qin P, Tan T. A novel medium poly(vinyl acetate-triallyl isocyanurate-divinylbenzene) coupled with oligo-β-cyclodextrin for the isolation of puerarin from pueraria flavones. Chromatographia. 2010;72:47–54. doi: 10.1365/s10337-010-1625-7. [DOI] [Google Scholar]
- 20.Connors KA. Binding Constants: The Measurement of Molecular Complex Stability. New York: Wiley; 1987. [Google Scholar]
- 21.Connors KA. The stability of cyclodextrin complexes in solution. Chem Rev. 1997;97:1325–1357. doi: 10.1021/cr960371r. [DOI] [PubMed] [Google Scholar]
- 22.Schneider H, Yatsimirsky A. Principles and Methods in Supramolecular Chemistry. Chichester: Wiley; 2000. [Google Scholar]
- 23.Paduano L, Sartorio R, Vitagliano V, Castronuovo G. Calorimetric and diffusional behaviour of the system α-cyclodextrin-L-phenylalanine in aqueous solution. Thermochim. Acta. 1990;162:155–161. doi: 10.1016/0040-6031(90)80337-X. [DOI] [Google Scholar]
- 24.Buckton G, Beezer AE. The application of microcalorimetry in the field of physical pharmacy. Int. J. Pharm. 1991;72:181–191. doi: 10.1016/0378-5173(91)90106-X. [DOI] [Google Scholar]
- 25.Yang L, Zhang H, Tan T, ur Rahman A. Thermodynamic and NMR investigations on the adsorption mechanism of puerarin with oligo-β-cyclodextrin-coupled polystyrene-based matrix. J. Chem. Technol. Biotechnol. 2009;84:611–617. doi: 10.1002/jctb.2089. [DOI] [Google Scholar]
- 26.Zhang H, Feng W, Li C, Tan T. Investigation of the inclusions of puerarin and daidzin with β-cyclodextrin by molecular dynamics simulation. J. Phys. Chem. 2010;114:4876–4883. doi: 10.1021/jp907488j. [DOI] [PubMed] [Google Scholar]
- 27.Adams B, Lerner LE. Effect of stereochemistry on hydroxyl proton chemical shifts and coupling constants in carbohydrates. Magn. Reson. Chem. 1994;32:225–230. doi: 10.1002/mrc.1260320407. [DOI] [Google Scholar]
- 28.Piotto M, Saudek V, Sklenar V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 29.Fielding L. Determination of association constants (Ka) from solution NMR data. Tetrahedron. 2000;56:6151–6170. doi: 10.1016/S0040-4020(00)00492-0. [DOI] [Google Scholar]
- 30.Bakkour Y, Vermeersch G, Morcellet M, Boschin F, Martel B, Azaroual N. Formation of cyclodextrin inclusion complexes with doxycyclin-hyclate: NMR investigation of their characterization and stability. J. Incl. Phenom. Macrocycl. Chem. 2006;54:109–114. doi: 10.1007/s10847-005-5108-7. [DOI] [Google Scholar]
- 31.Stauffer DA, Barrans RE, Dougherty DA. Concerning the thermodynamics of molecular recognition in aqueous and organic media. Evidence for significant heat capacity effects. J. Org. Chem. 1990;55:2762–2767. doi: 10.1021/jo00296a038. [DOI] [Google Scholar]
- 32.Cameron KS, Fielding L. NMR diffusion spectroscopy as a measure of host-guest complex association constants and as a probe of complex size. J. Org. Chem. 2001;66:6891–6895. doi: 10.1021/jo010081x. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Tan T, Kenne L, Sandström C. The use of diffusion-ordered spectroscopy and complexation agents to analyze mixture of catechins. New J. Chem. 2009;33:1057–1063. doi: 10.1039/b900164f. [DOI] [Google Scholar]
- 34.Bekiroglu S, Kenne L, Sandström C. 1H NMR studies of maltose, maltoheptaose, α-, β-, and γ-cyclodextrins, and complexes in aqueous solutions with hydroxy protons as structural probes. J. Org. Chem. 2003;68:1671–1678. doi: 10.1021/jo0262154. [DOI] [PubMed] [Google Scholar]
- 35.Bendeby B, Kenne L, Sandström C. 1H-NMR studies of the inclusion complexes between α-cyclodextrin and adamantane derivatives using both exchangeable hydroxy protons and non-exchangeable aliphatic protons. J. Incl. Phenom. Mol. Recognit. Chem. 2004;50:173–181. doi: 10.1007/s10847-004-8952-y. [DOI] [Google Scholar]
- 36.Bekiroglu S, Sandström A, Kenne L, Sandström C. Ab initio and NMR studies on the effect of hydration on the chemical shift of hydroxy protons in carbohydrates and water/methanol/ethers as model systems. Org. Biomol. Chem. 2004;2:200–205. doi: 10.1039/b311852e. [DOI] [PubMed] [Google Scholar]
- 37.Sandström C, Baumann H, Kenne L. The use of chemical shifts of hydroxy protons of oligosaccharides as conformational probes for NMR studies in aqueous solution. Evidence for persistent hydrogen bond interaction in branched trisaccharides. J. Chem. Soc., Perkin Trans. 1998;2:2385–2393. [Google Scholar]
- 38.Xu J, Tan T, Janson J-C, Kenne L, Sandström C. NMR Studies on the interaction between (-)-epigallocatechin gallate and cyclodextrins, free and bonded to silica gels. Carbohydr. Res. 2007;342:843–850. doi: 10.1016/j.carres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Poppe L, Halbeek H. Nuclear magnetic resonance of hydroxyl and amido protons of oligosaccharides in aqueous solution: evidence for a strong intramolecular hydrogen bond in sialic acid residues. J. Am. Chem. Soc. 1991;113:363–365. doi: 10.1021/ja00001a055. [DOI] [Google Scholar]
- 40.Yang L, Zhu Y, Tan T, Janson J-C. Coupling oligo-β-cyclodextrin on polyacrylate beads media for separation of puerarin. Process Biochem. 2007;42:1075–1083. doi: 10.1016/j.procbio.2007.04.008. [DOI] [Google Scholar]
- 41.Nørbæk R, Brandt K, Kondo T. Identification of flavone C-glycosides including a new flavonoid chromophore from barley leaves (Hordeum vulgare L.) by improved NMR techniques. J. Agric. Food Chem. 2000;48:1703–1707. doi: 10.1021/jf9910640. [DOI] [PubMed] [Google Scholar]
- 42.Caristi C, Bellocco E, Panzera V, Toscano G, Vadalà R, Lezzi U. Flavonoids detection by HPLC-DAD-MS-MS in lemon juices from Sicilian cultivars. J. Agric. Food Chem. 2003;51:3528–3534. doi: 10.1021/jf0262357. [DOI] [PubMed] [Google Scholar]
- 43.Rayyan S, Fossen T, Nateland HS. Isolation and identification of flavonoids, including flavone rotamers, from the herbal drug ‘crataegi folium cum flore’ (hawthorn) Phytochem. Anal. 2005;16:334–341. doi: 10.1002/pca.853. [DOI] [PubMed] [Google Scholar]