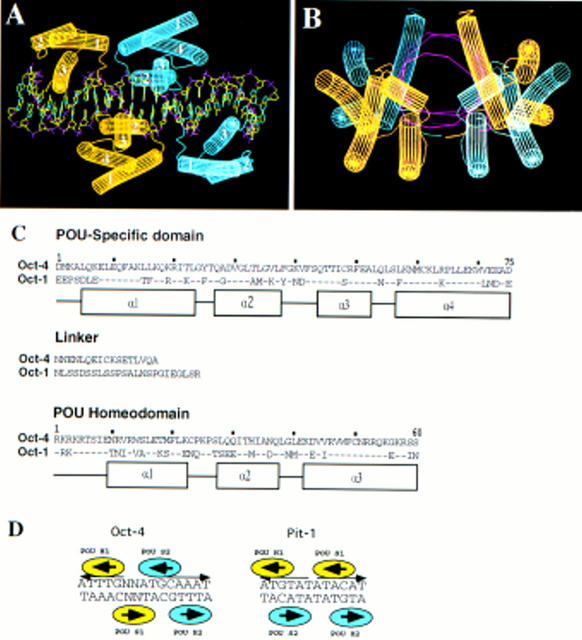
Figure 8.
Computer modeling of Oct-4 POU domain dimer conformation on the PORE binding sequence. (A,B) Lateral and frontal view of Oct-4 POU domain dimer conformation on the PORE binding site. Oct-4 POU domains were modeled into the coordinates of Oct-1. Each Oct-4 molecule has one color. The POUS domain has four α-helices (labeled 1–4) and the POUHD has three α-helices (labeled 1–3). DNA structure is represented by purple for phosphate, red for oxygen, green for carbon, and blue for nitrogen. A and B generated with WHAT IF software. (C) Amino acid sequence alignment of POU specific, linker, and POU homeodomain of Oct-1 and Oct-4. Amino acids numeration are taken from Herr and Cleary (1995). Dots represents identical amino acids found in Oct-4. α-Helices are indicated by boxes. (D) Schematic representation of Oct-4 and Pit-1 homodimers. Each molecule is given one color.