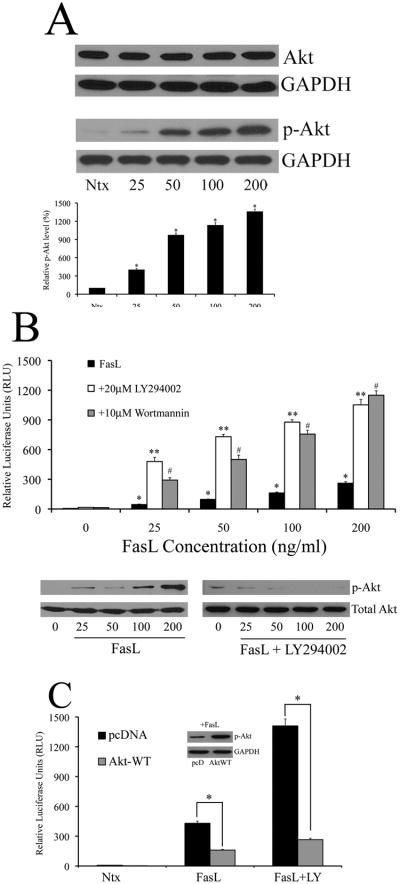
Fig. 2. FasL activates PI3K/Akt in a dose-dependent manner – Akt negatively regulates NF-κB.
A. 293T cells were treated with increasing doses of FasL (25–200ng/ml) or left in serum free medium (SFM) alone without FasL (referred to as “Ntx” here and henceforth in the figures) for 1 h and assayed for total Akt and phosphorylated Akt-473 levels by immunoblot analysis. Blots were re-probed with GAPDH antibody to confirm equal loading of the samples. The immunoblot signals for phosphorylated Akt were quantified by densitometry. B. Cells co-transfected with the NF-κB-Luc and pRL-tk vectors were either left untreated or pre-treated with 20μM LY294002 or 10μM wortmannin for 1 h. Following pre-treatment, cells were treated with FasL (0–200ng/ml) for 12 h, and assayed for NF-κB activity. Concurrently, cells were lysed and assayed for phosphorylated Akt to confirm modulation of Akt using western blotting. Blots were reprobed with GAPDH antibody to confirm equal loading of samples. C. In addition to the luciferase vectors described in A, cells were co-transfected with either 1μg of control pcDNA3 plasmid or constitutively active Akt plasmid (Akt-WT). Post transfection, cells were treated with 200ng/ml FasL for 12 h in the presence or absence of 20μM LY294002 (1 h pre-treatment) and assayed for NF-κB activity by luciferase assay. Further, FasL-treated lysates were probed for phosphorylated Akt to confirm effect of Akt-WT plasmid. Plots show relative NF-κB levels over non-treated control. Values are mean ± SD (n = 4). *P < 0.05 versus non-treated control. **P < 0.05, #P < 0.05 versus FasL-treated datasets.