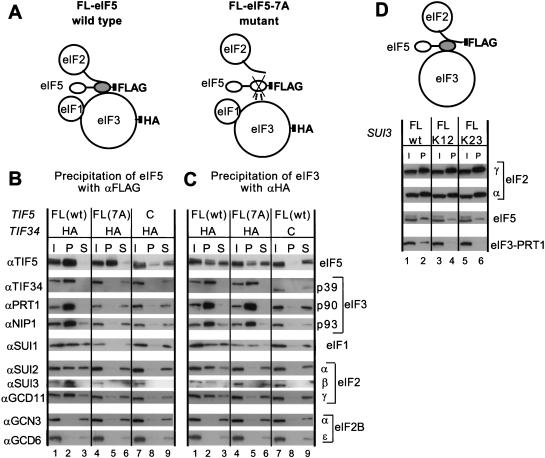
Figure 2.
Coimmunoprecipitation of eIF2 and eIF3 from cell extracts dependent on AA-box 2 in the carboxyl terminus of eIF5. (A) Schematic model of the structures of the multifactor complexes found in KAY50 and KAY51 (Table 1) containing wild-type eIF5-FL (left) or eIF5-FL-7A (right), as deduced from this study. The modular structure of eIF5 is represented by two ovals (amino-terminal and carboxy-terminal domains) connected with a thick line (less conserved region). The shaded oval in eIF5 corresponds to the B6 fragment, necessary and sufficient for binding to eIF2 and eIF3 (Asano et al. 1999). This oval is crossed in the mutant, indicating the 7A mutation in AA-box 2. The curved line attached to eIF2 represents the amino-terminal half of eIF2β containing the K-boxes which interact with the carboxy-terminal domain of eIF5. Filled squares indicate the epitope tags on eIF3-HA-TIF34 or FL-eIF5. Dotted lines indicate a weakened interaction. (B, C) Coimmunoprecipitation of eIFs with eIF5-FL or TIF34-HA. WCEs were prepared from strains KAY50, KAY51, KAY10, and KAY37 (Table 1), grown in YPD medium at 30°C. Aliquots of WCEs were incubated with anti-FLAG (B) or anti-HA (C) affinity resin and after extensive washing, the bound proteins were analyzed by SDS-PAGE and immunoblotting using the antibodies indicated on the left. Lanes 1, 4, and 7, 20% input (I) amounts of WCE; lanes 2, 5, and 8, the entire precipitated (P) fractions; lanes 3, 6, and 9, 10% of supernatant (S) fractions. (D) Coimmunoprecipitation of eIFs with eIF2β-FL. The schematic model is similar to that described in B and C except that eIF2β carries the FLAG epitope. WCEs prepared from strains KAY33 (SUI3), KAY25 (SUI3-FL), KAY29 (sui3-FL-K12) and KAY30 (sui3-FL-K23) (Asano et al. 1999) were immunoprecipitated with FLAG affinity resin and the precipitated proteins were analyzed by immunoblotting using the appropriate antibodies. (I) 20% input amount of WCE (lanes 1, 3, and 5); (P) the entire precipitated fractions (lanes 2, 4, and 6). K12 and K23 indicate the Ala substitutions present in K-boxes 1–2 or 2–3, respectively, in the mutant eIF2β-FL proteins.