Figure 3.
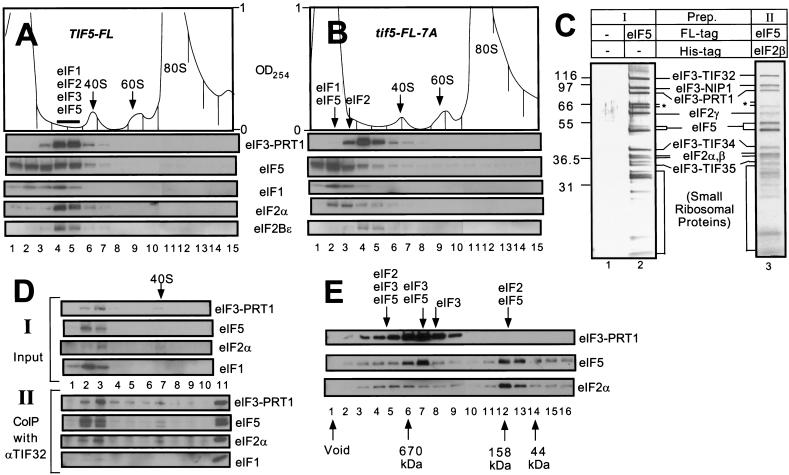
The multifactor complex containing eIF1, eIF2, eIF3, and eIF5 can be isolated free of 40S ribosomes. (A,B) Twenty A260 units of WCEs prepared from KAY50 (TIF5-FL) (A) and KAY51 (tif5-FL-7A) (B) were resolved on 15%–40% sucrose gradients by centrifugation for 4.5 h at 39,000 rpm. Gradients were separated into 20 0.6 mL-fractions while scanning continuously at A254 as shown ( top panels). Portions of the fractions were precipitated with 10% TCA and analyzed by SDS-PAGE and immunoblotting using antibodies against the factors listed beside the five bottom panels. (C) Silver staining of purified preparations of the multifactor complex. The staining patterns of Type I (lane 2) and Type II (lane 3) preparations are shown along with a mock preparation obtained from a strain containing untagged eIF5 (lane 1) in parallel with the Type I preparation. (*) Degradation products of TIF32. (D) Size fractionation and coimmunoprecipitation analysis of a Type II preparation of the multifactor complex. A sample containing 0.5 mL (50 μg protein) of Type II preparation was resolved on 7.5%–30% sucrose gradient by centrifugation for 5 h at 41,000 rpm. The gradient was separated into 10 1.2 mL-fractions while scanning continuously at A254. One hundred μL of each fraction were precipitated with 10% TCA (top panels, input), 400 μL were immunoprecipitated with anti-TIF32 antibodies (bottom panels, CoIP with αTIF32), and the resulting samples were analyzed by immunoblotting using antibodies against the factors listed to the right. The position of the 40S ribosomes is indicated. (Lane 11) 1% of the Type II preparation separated on the sucrose gradient. (E) Size fractionation of Type I preparation of the multifactor complex. Two hundred microliters of Type I preparation was separated on a Superose 6 sizing column (Pharmacia), preequilibrated with buffer A without pepstatin, aprotinin, and leupeptin, using the FPLC system (Pharmacia), as described previously (Phan et al. 1998). Portions of the fractions were precipitated with 10 % TCA, and analyzed by SDS-PAGE and immunoblotting using antibodies against the factors listed on the right. Lane 1: sample from the void volume. Arrows below the panels indicate the elution positions of size standards (BioRad) determined in a parallel experiment. Arrows above the panels indicate the positions of different complexes deduced from the immunoblot analysis.