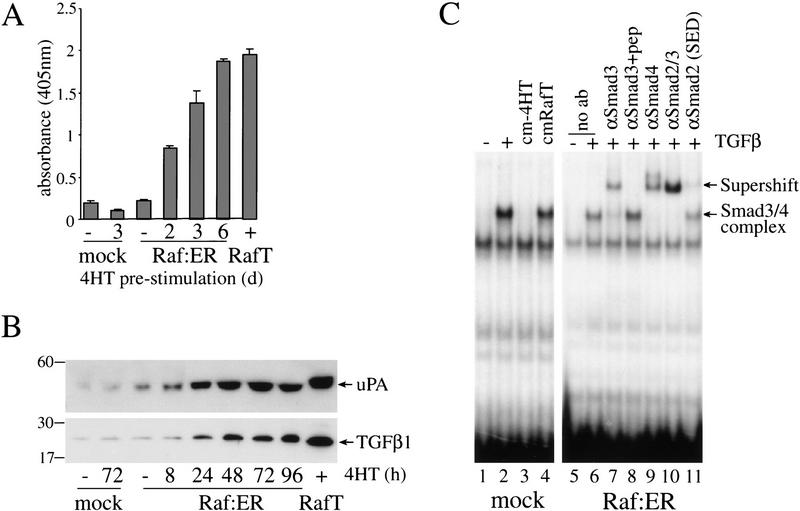
Figure 3.
Activation of Raf in MDCK cells induces secretion of TGFβ. (A) MDCK Raf–ER cells, control MDCK cells, and MDCK Raf–ER cells transformed by long-term culture in 4HT (Raf T) were cultivated in the presence or absence of 100 nM 4HT in DMEM +2% FCS for 24 h. Cells were cultured in DMEM +0.5% BSA for a further 24 h before collecting the supernatants. Cell culture supernatants were examined for TGFβ1 levels by ELISA. The values shown were normalized for cell number. (B) MDCK Raf–ER cells, control MDCK cells, and Raf-transformed MDCK Raf–ER cells (RafT) were cultured in DMEM +2% FCS and stimulated with 100 nM 4HT for the indicated times. The cell culture supernatant was concentrated by ultrafiltration and equal aliquots were analyzed by 12% SDS-PAGE under nonreducing conditions and immunoblotted for TGFβ and Urokinase-type Plasminogen Activator (uPA). (C) MDCK control cells were either untreated or treated for 1 h with 2 ng/mL TGFβ, with conditioned medium of 4HT untreated MDCK Raf–ER cells (cm-4HT) or with conditioned medium of MDCK Raf–ER cells transformed by long-term exposure to 4HT (cmRafT). Nuclear extracts were assayed for the binding of activated Smad3/4 complexes to a 32P-labeled c-jun oligonucleotide probe by EMSA (lanes 1–4). Untreated MDCK Raf–ER cells were stimulated with 2 ng/mL TGFβ for 1 h, and nuclear extracts were assayed in supershifts for the presence of Smads in complexes bound to the c-jun probe with the following antibodies: anti-Smad3, anti-Smad4, anti-Smad2, cross-reacting with Smad3 (Transduction Laboratories), and anti-Smad2 (SED). Peptide competition was performed with anti-Smad3 (Smad3 +pep).