Figure 5.

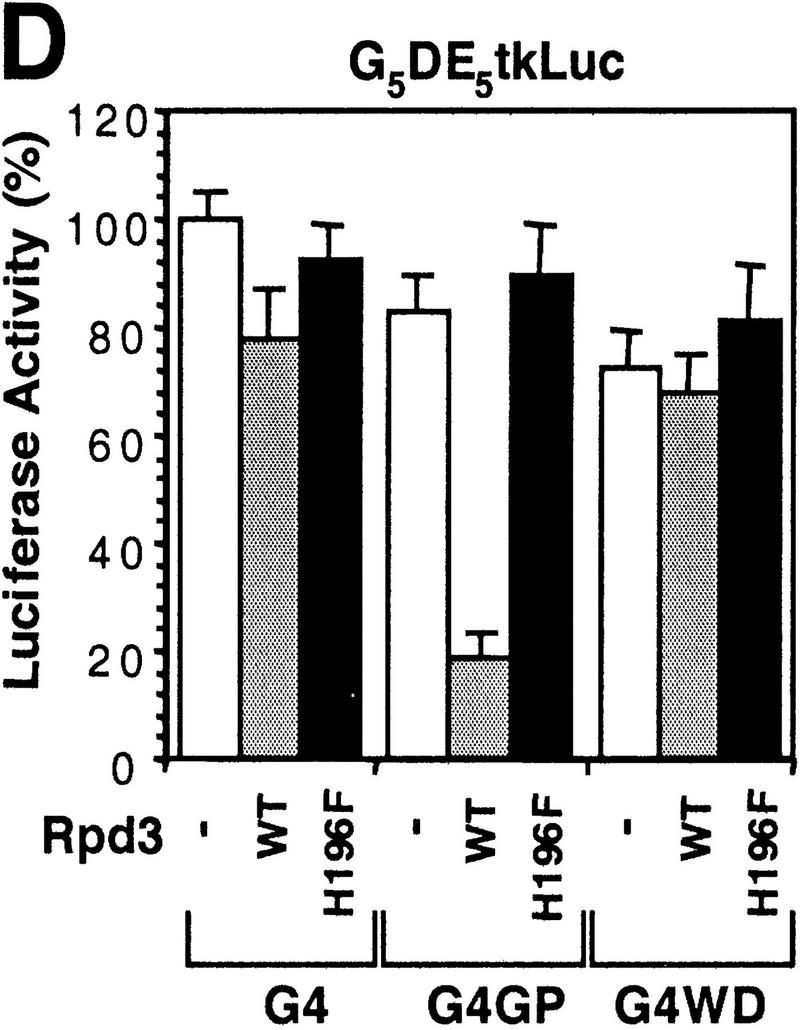
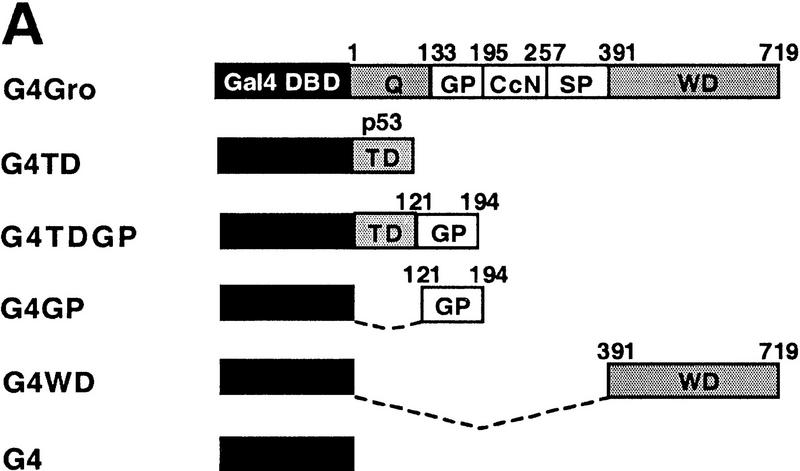
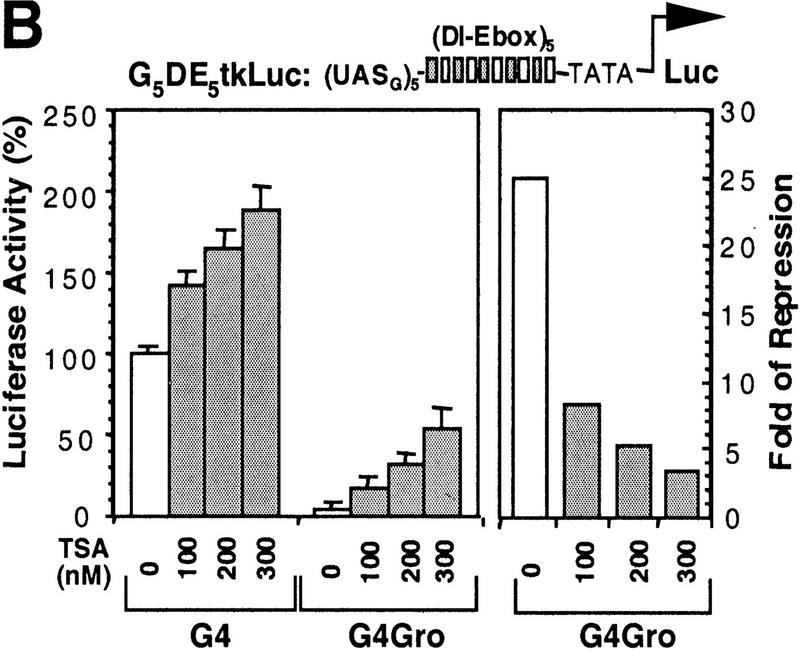
Histone deacetylase activity contributes to Gro-mediated repression in cultured cells. (A) Schematic diagram of various Gal4–Gro fusion constructs used in the cotransfection assays described in B–D. (TD) The tetramerization domain of p53 (residues 309–371). (B) TSA treatment dramatically reduces Gal4–Gro mediated repression. The structure of the firefly luciferase reporter (G5DE5tkLuc) is depicted at top. This reporter and an internal control reporter (p-37tkRLuc) encoding Renilla luciferase were cotransfected into S2 cells with vectors expressing Dorsal, Twist, and the Gal4 DNA-binding domain (G4) or the Gal4–Gro fusion protein (G4Gro). Twenty-four hr post-transfection, cells were treated with the specified amounts of TSA and luciferase activities were measured 10 hr later. All firefly luciferase activities (normalized first to the control Renilla luciferase activities) are normalized to the activity in the presence of Dorsal, Twist, and Gal4 DBD alone, which is set at 100%. Each bar represents the average plus standard deviation of three independent duplicate assays (left). The fold repression (right) reflects the ratio of the activity observed with Gal4 DNA-binding domain alone to that observed with Gal4–Gro at each TSA concentration. (C) The Gro GP domain is able to repress transcription when fused to the Gal4 DBD and the p53 TD (G4TDGP). However, the GP domain alone (G4GP) and the p53 TD alone (G4TD) fail to repress transcription when fused to the Gal4 DNA-binding domain. Cotransfection assays were conducted as described in B. (D) The Gal4–GP fusion protein synergizes with the enzymatically active form of Rpd3 to repress transcription. S2 cells were transfected with the reporters and expression constructs encoding Dorsal, Twist, and the indicated Gal4 fusion protein in the absence (−, open bars) or presence of a vector expressing either wild-type (WT, shaded bars) or single-point mutant (H196 F, solid bars) forms of Rpd3.