Fig. 3.
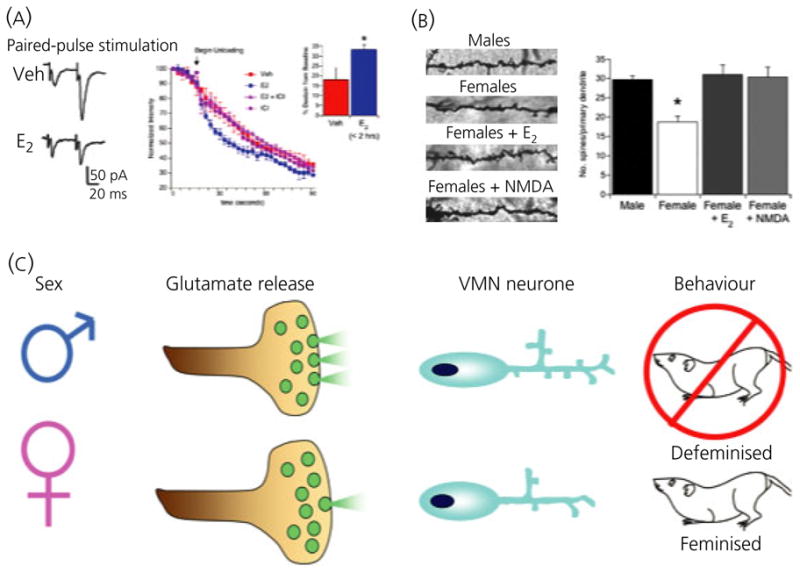
Nongenomic steroid-induced organisation of the hypothalamus. Oestradiol rapidly induces glutamate release from hypothalamic neurones leading to activation of post-synaptic glutamate receptors and induction of dendritic spines. (A) Paired pulse stimulation ratios indicate enhanced release of glutamate on the second stimulation in neurones pretreated with oestradiol. The rate of destaining of FM4-64 visualised terminals confirms this, and the effect of oestradiol is blocked by the highly specific oestrogen receptor antagonist, ICI 182,780. The effect of oestradiol on FM4-64 destaining is apparent within 1 h of treatment and does not require protein synthesis but does require activation of phosphoinositide 3-kinase (PI3) kinase. (B) Golgi impregnated ventromedial nucleus (VMN) neurones reveal a higher number of dendritic spines on the neurones of males or females treated with oestradiol compared to control females (*P < 0.05 compared to all other groups, ANOVA). Treatment with the glutamate agonist, NMDA, mimics the effect of oestradiol, confirming an essential role for glutamate transmission in establishing sexually dimorphic dendritic morphology (25). (C) Schematic representation of the role of glutamate in defeminisation. Enhanced glutamate release in male neurones results in increased dendritic spines, which is correlated with a loss of expression of female sexual behaviour expression in adulthood. Treatment of neonatal females with NMDA successfully defeminises their adult behaviour, further confirming the role of glutamate in the organisational component of sexual differentiation.
