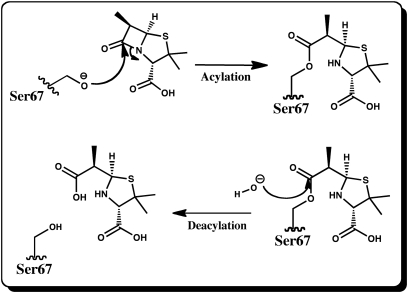
Fig. 1.
Mechanism for the acylation and deacylation reactions of Class D β-lactamases. In the first half-reaction nucleophilic Ser residue attacks the carbonyl carbon of the β-lactam ring leading to cleavage of the ring and formation of a stable doripenem-enzyme acylate. In the second step, a water molecule attacks the same carbon, the Ser-doripenem bond breaks and the inactivated ligand is released from the active site.