Figure 4.
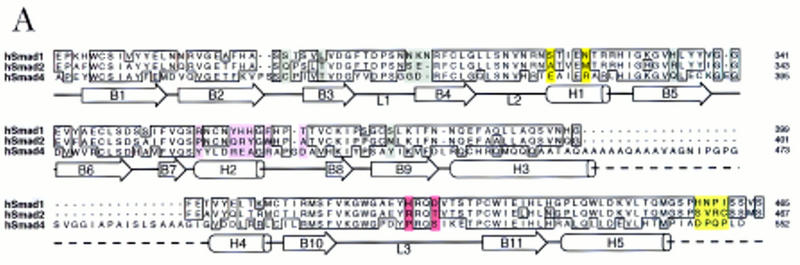
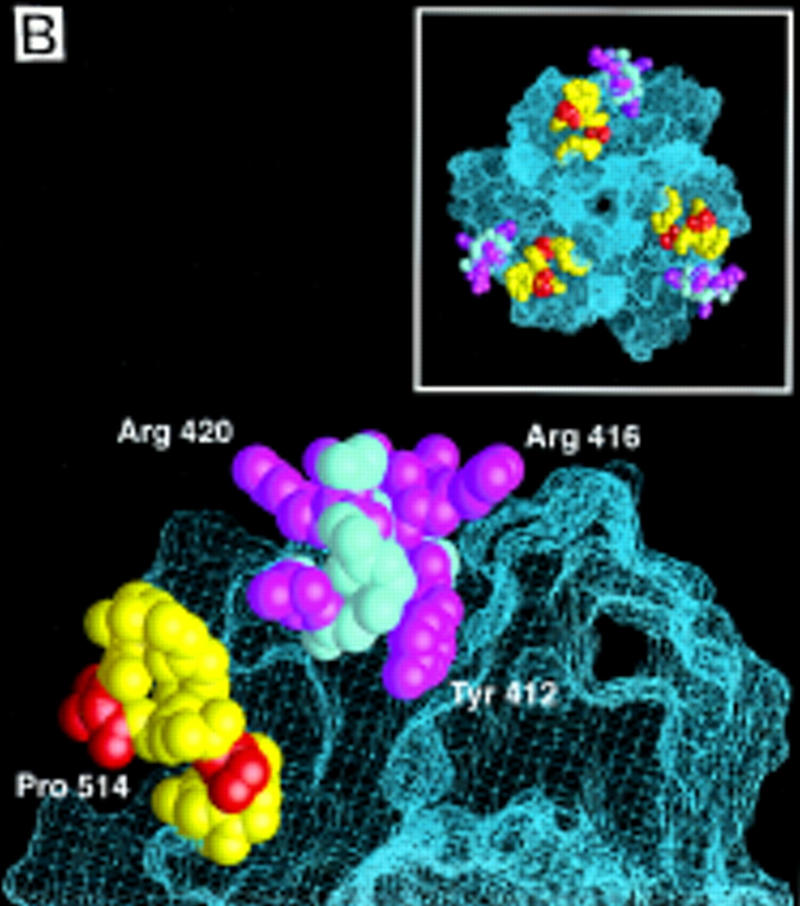
(A) Sequence alignment of the MH2 domains of Smad1, 2, and 4, with the Smad4 MH2 domain secondary structure elements indicated below. Identical residues are boxed. Subtype-specific residues map to α-helix 1 (yellow), α-helix 2 and its vicinity (purple), the L3 loop (red), and immediately upstream of the carboxy-terminal receptor phosphorylation motif SS(V/M)S (green). The remaining subtype-specific residues (gray) are scattered in the primary sequence but clustered in the crystal structure near the point of connection to the amino-terminal half of the molecule (Shi et al. 1997). (B) A close-up, lateral view of the Smad4 MH2 crystal structure showing the L3 loop (yellow) with subtype specific residues (red) and the α-helix 2 (cyan) with subtype-specific residues (magenta). (Inset) Frontal view of the location of the L3 loop and helix 2 of each MH2 monomer in the crystallographic trimer.
