Table 1.
Homocoupling of aryl halides using the integrated flow microreactor system.
| Ar–X | T (°C) | tR1 (s) | Ar–Ar | Yield (%) |
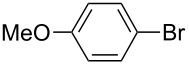 |
24 | 3.100 | 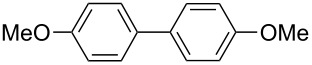 |
72 |
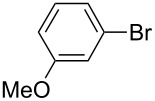 |
24 | 3.100 | 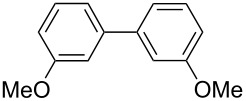 |
69 |
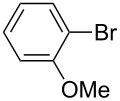 |
0 | 3.100 | 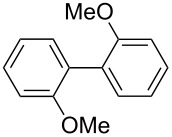 |
76 |
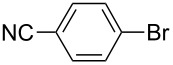 |
−28 | 0.055 | 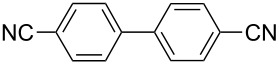 |
75 |
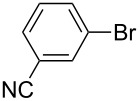 |
0 | 0.055 | 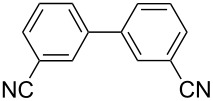 |
66 |
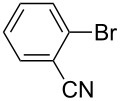 |
24 | 0.055 | 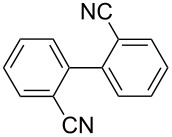 |
76 |
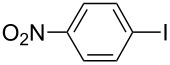 |
−48 | 0.014 | 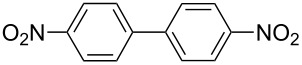 |
53a |
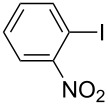 |
−48 | 0.014 | 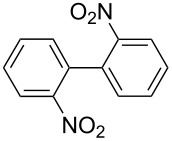 |
63a |
aPhLi instead of n-BuLi was used as lithiating reagent.
