Figure 2.
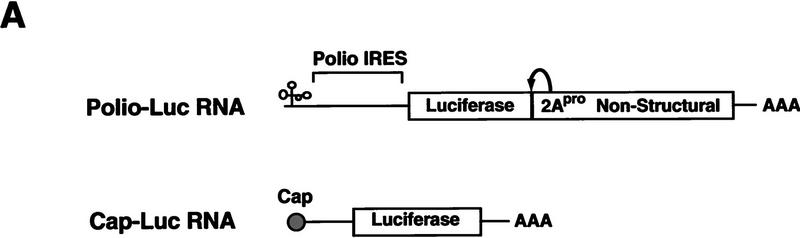
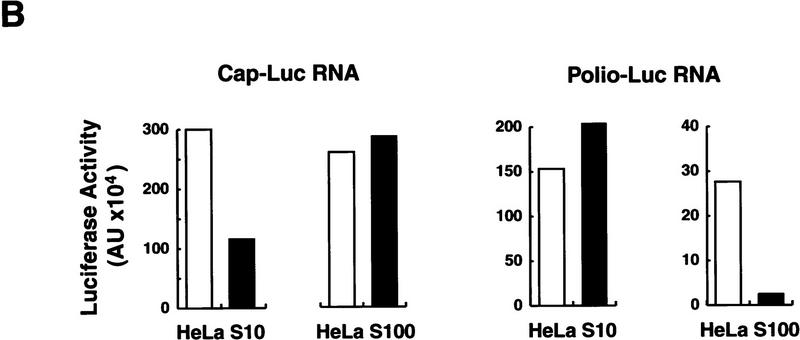
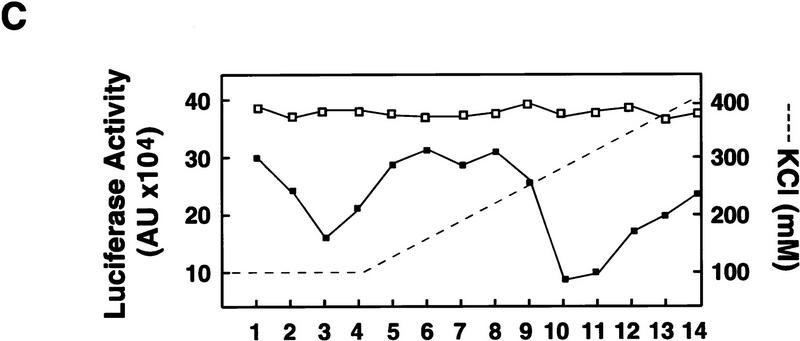
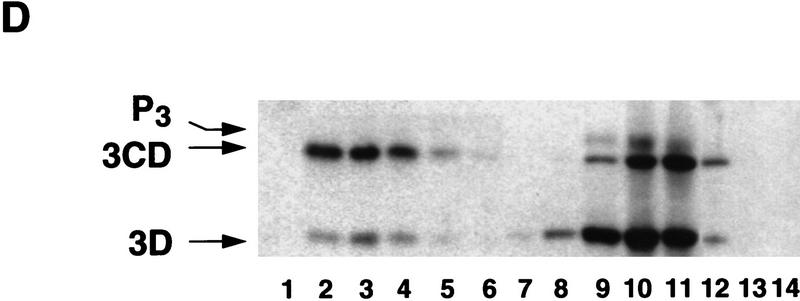
Poliovirus-infected cell extracts contain an activity that specifically inhibits poliovirus translation. (A) Schematic representation of the chimeric poliovirus luciferase RNA (Polio–Luc) and capped luciferase RNA (Cap–Luc). In Polio–Luc, the coding region of the poliovirus capsid proteins was replaced by the luciferase reporter gene, and a cleavage site for 2Apro has been introduced between luciferase and 2Apro (represented by the arrow). The Cap–Luc RNA consists of the luciferase gene flanked by the 5‘ and 3‘ uncoding regions of the β-globin mRNA. (B) Microinjection of infected S100 HeLa cell extract into Xenopus oocytes specifically inhibits poliovirus cap-independent translation. Polio–Luc or Cap–Luc RNA was injected into oocytes together with uninfected (open bars) or poliovirus-infected S10 or S100 HeLa cell fractions (solid bars) as indicated in each case. Luciferase activity was determined in oocytes after 3 hr of incubation at 22°C and expressed in arbitrary units (AU). (C) Elution profile of the translation inhibitory activity after ion-exchange chromatography. Infected S100 HeLa cell extract was loaded onto a HiTrap SP column (Pharmacia) and eluted with a KCl gradient, as indicated at right. The translation inhibitory activity was determined by coinjection of 20 nl of each fraction (1–14) together with 5 nl of HeLa S10 (to provide the cellular factor essential for poliovirus translation in oocytes, PTF) and 20 ng of Polio–Luc RNA (▪) or Cap–Luc RNA ( ) into oocytes. Luciferase activity was determined in oocyte extracts after 3 hr of incubation at 22°C and expressed in AU. (D) Viral proteins 3Dpol, 3CD, and P3 copurified with the viral translation inhibitory activity. Western blot analysis of fractions 1–14 eluted from the HiTrap SP column is shown. Two microliters of each fraction was resolved in a 10% SDS–polyacrylamide gel, transferred to nitrocelluose membrane, and probed with specific anti-3CD antibodies. The electrophoretic mobility of P3, 3CD, and 3D is indicated at left.