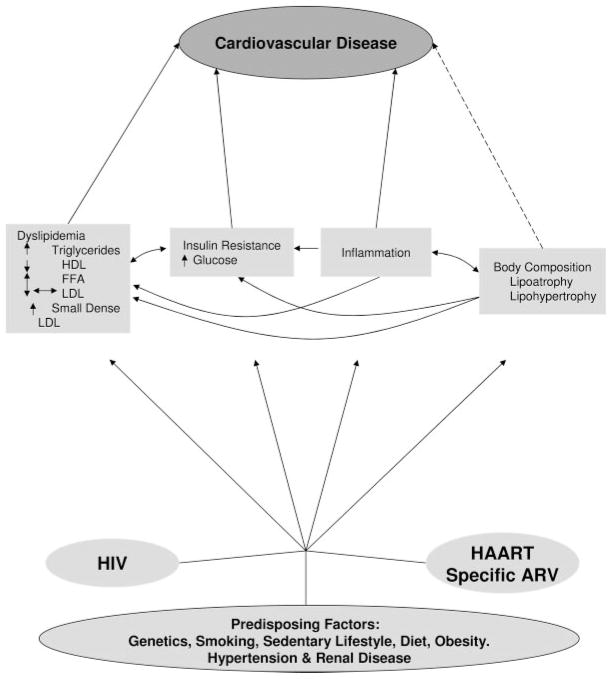
Patients with human immunodeficiency virus (HIV) infection have sustained alterations in metabolism (lipids and insulin/glucose homeostasis) and body composition (fat distribution) that are proatherogenic (the Figure). HIV infection itself and/or its therapies may contribute to these alterations (the Table); although most effects are reversible, there are some possibly irreversible consequences of treatment. With the relative restoration to health seen in the era of highly active antiretroviral therapy (HAART), many traditional risk factors and promoters of dyslipidemia and diabetes also are present; they interact with HIV-specific inducers to worsen dyslipidemia and to increase the prevalence of insulin resistance and diabetes.
Figure.
Overview of the effects of HIV and its therapies on CVD risk. The contribution of traditional risk factors must be kept in mind, and they may occur with increased prevalence in people with HIV infection (eg, smoking). HIV, likely through the inflammatory response, and antiretroviral therapies independently affect many of the mediators of CVD risk. The effects on lipids are a prominent but complex example; HIV infection lowers LDL levels, but antiretroviral therapy raises LDL back up to normal levels. The bidirectional arrows indicate associations, but there is not yet adequate proof of causality. The dotted arrow between body composition and CVD indicates that body fat is known to affect the mediators such as dyslipidemia and insulin resistance but may also have a direct effect. FFA indicates free fatty acids; ARV, antiretroviral.
Table.
Effects of HIV Treatment
| Effects of Untreated or Ineffectively Treated HIV | Changes Associated With Viral Suppression | Changes With Specific Antiretroviral Therapy | Role of Body Shape Changes | |
|---|---|---|---|---|
| Lipid metabolism | ||||
| HDL-C | Decreases early in infection | Increases modestly but not to premorbid levels | Greater increases seen with NNRTI and possibly atazanavir than other PI | Increased VAT and upper trunk fat associated with low HDL-C |
| LDL-C | Decreases later in infection | Increases modestly | No evidence of direct drug effects | |
| Triglycerides | Increases in late infection | Decreased in early study of AZT monotherapy | No decrease or even further increase with HAART regimens containing ritonavir and other (but not all) PIs; increases also may be seen with stavudine and efavirenz | Increased VAT and upper trunk fat but lower leg fat associated with increased triglyceride levels |
| Glucose metabolism | ||||
| Insulin sensitivity | Some early evidence of increase; recent data find decrease | Overall trend to decreased insulin sensitivity independently of regimen; some insulin resistance represents restoration to health | Some PIs decrease; NRTIs (particularly stavudine) also may contribute to decreases | Increased VAT and upper trunk fat and lower limb fat associated with insulin resistance |
| Insulin secretion | No evidence of an effect | No evidence of an effect | Some PIs may decrease insulin secretion | |
| Fasting glucose | No evidence of an effect | Increased in some studies; some PIs increase endogenous glucose production | ||
| Glucose tolerance | No evidence of an effect | Higher rates of IGT | ||
| Diabetes | No evidence of differences in incidence or prevalence | Possibly higher prevalence of diabetes | Higher prevalence and incidence of type 2 diabetes; associated with some PI (especially indinavir, ritonavir) and NRTI (especially stavudine) | |
| Body composition | ||||
| Lean body mass | Decreases disproportionately with severe wasting | Increases modestly with initiation of effective HAART | No consistent evidence of direct drug effects | |
| Peripheral fat | Decreases proportionally with wasting | Initially increases with initiation of effective HAART | Subsequent preferential depletion of subcutaneous fat in face, arms, legs, and buttocks more than upper trunk associated with some NRTIs (especially, thymidine analogues); role of different PIs and NNRTIs unclear | |
| Visceral fat | Decreased but possibly relatively preserved; effect of weight cycling during episodic weight loss and regain unknown | Increases with initiation of effective HAART | Preserved or increased visceral fat in some patients on HAART; association with specific drugs or drug classes unclear | |
| Renal function | ||||
| Renal disease | HIVAN | Decreased HIVAN | Some impaired renal function with tenofovir | |
| Microalbuminuria and elevated cystatin C | Microalbuminuria and elevated cystatin C remain common in current era | |||
PI indicates protease inhibitors; AZT, azidothymidine; IGT, impaired glucose tolerance; and HIVAN, HIV-associated nephropathy.
These disturbances in lipid and glucose metabolism and renal disease may contribute, at least in part, to the excess cardiovascular disease (CVD) morbidity and mortality observed in HIV-infected individuals (the Figure). However, the relative contribution to excess CVD risk of traditional CVD risk factors, especially smoking, compared with these infection- and treatment-specific complications requires clarification. More prospective data with multivariable modeling are needed.
Dissecting the Effect of HIV Alone: Metabolic Parameters and Body Composition Before HAART
Lipid and Lipoproteins
High-density lipoprotein cholesterol (HDL-C) levels decrease early in HIV infection1,2 (the Table). Low-density lipoprotein cholesterol (LDL-C) decreases slightly later.1,2 Subsequently, triglycerides and very low–density lipoprotein (VLDL) cholesterol (VLDL-C) increase, often at the time when signs and symptoms of AIDS occur.1,2 The relative proatherogenic effect of reduced HDL may exceed the protective effect of the slight reduction in LDL; the increase in VLDL-C may offset the decrease in LDL-C. HIV infection frequently is characterized by hypertriglyceridemia resulting from increased VLDL. Under those circumstances, non–HDL-C is likely a better predictor of CVD risk than LDL-C.
These lipid changes are related to indexes of HIV disease stage and activity such as increasing HIV RNA levels and decreasing CD4 counts.1–3 Likely mediators are cytokines involved in the host response to infection. Although multiple cytokines may be involved, the strongest link is between interferon-α levels and the changes in triglyceride metabolism.4 Triglyceride clearance is slowed and de novo lipogenesis is increased, 2 changes that correlate with interferon-α levels.1,5 Free fatty acids may rise, but the contribution of increased free fatty acids to the HIV-induced increase in triglycerides is not clear.
Importantly, lipoprotein composition also changes. There are increased concentrations of small dense LDL,6 which is thought to penetrate vessel walls and undergo oxidation more easily.7 Oxidized LDL is more atherogenic than nonoxidized LDL.8 Circulating levels of oxidized LDL may increase.9,10 Increased levels11 are seen of the enzyme plasma platelet-activity factor (PAF) acetylhydrolase, which cleaves oxidized phosphatidylcholine to lysophosphatidylcholine, a lipid that promotes macrophage recruitment into the vessel wall.8 More study is needed on the fine structure of lipoproteins that might influence atherosclerosis risk independently of lipoprotein levels. Early evidence suggests that HIV-infected macrophages may be more susceptible to foam cell formation, an early step in atherosclerosis.12
Although many of these changes might be predicted to increase atherosclerosis, in aggregate, there is little evidence to permit estimation of the contribution of individual alterations to atherosclerotic disease. Indeed, the contribution of untreated HIV infection to CVD is debated. For example, of the 2 largest studies, 1 study found that the incidence of CVD events decreased at the time of introduction of effective HAART, and the other found an increase.13,14 In a randomized trial of treatment to suppress HIV RNA compared with intermittent therapy to maintain CD4 cell counts, intermittent therapy had more complications, including increased CVD, suggesting that sustained therapy reduced CVD.15
Glucose Metabolism and Insulin Resistance
Interpreting published studies to provide a unifying summary of the effects of HIV infection per se on insulin sensitivity is difficult because there are conflicting results from current studies of antiretroviral-naive subjects and earlier studies of naive or ineffectively treated subjects (the Table). In early studies before the availability of effective HAART when AIDS wasting was common, patients were not insulin resistant, and evidence of disturbances of glucose metabolism was unusual.16,17 More recently, insulin resistance may be seen before the initiation of HAART.18
Explanations for this discrepancy may relate to differences in the body composition characteristics of the 2 eras (see the Body Composition section below). In early studies before HAART, patients were cachectic or had a body mass index (BMI) <25 kg/m2 and thus did not have a phenotype prone to insulin resistance and disorders of glucose metabolism.19 In the current era, there may be a greater prevalence of visceral adipose tissue (VAT) and even obesity at the time of HIV diagnosis or initiation of treatment20; obesity is associated with insulin resistance.21
Body Composition
As discussed above, historically, many HIV-infected patients became cachectic or at least had lower BMIs (<25 kg/m2) than their non–HIV-infected counterparts19 (the Table). Negative energy balance (decreased energy intake resulting from opportunistic infection or gastrointestinal disease plus inappropriately increased resting energy expenditure resulting from HIV) caused weight loss, followed by failure to return to preillness weight.22–25
Early studies of cachexia suggested that lean mass was lost preferentially.19,26,27 In later studies, weight loss was accompanied by more loss of fat than of lean.28,29 However, in retrospect, one cannot rule out nucleoside reverse transcriptase inhibitor (NRTI)–induced fat loss as a contributor to the later results.30
Extrapolation from studies in other diseases and limited studies in HIV suggests conservation of VAT relative to subcutaneous adipose tissue before treatment with HAART.20,31,32 Many studies in HIV infection include young and female subjects who have little VAT, making depletion or conservation of VAT hard to assess. Weight loss in obesity may have preferential VAT loss, which is accompanied by improvements in metabolic CVD risk factors.21,33 However, when total fat is significantly depleted in any disease, VAT may be relatively preserved compared with subcutaneous adipose tissue during weight loss.31,32 The metabolic and health consequences of depot-specific fat loss in the context of diminished lean tissue mass are not yet fully understood. Little is known about the interaction of aging with these disease-related changes.
The current patterns of body composition found in research studies differ from the pattern in the pre-HAART era for a variety of reasons, particularly changing demographics. Patients are starting HAART with higher BMI and body fat mass, if not obesity.20 HIV-infected people may be healthier and heavier at initiation of HAART, whereas earlier research studies often focused on people with recent opportunistic infections who had illness-related wasting and included predominantly middle class, homosexual white men. Now, studies of HIV infection include individuals of both genders and all races and within various social classes who have genetic and/or environmental factors (socioeconomic status, diet, smoking, sedentary lifestyle) that predispose to obesity, increased VAT, and insulin resistance.34 On the other hand, women more often have gluteofemoral fat accumulation rather than the abdominal/truncal accumulation that is the typical pattern found in men.35 More gluteofemoral fat also is found in blacks among both men and women, in contrast to whites and Hispanics.35 Abdominal truncal fat is more associated with metabolic abnormalities. It should be recognized that in Asians, higher levels of VAT are present for corresponding lower weight, BMI, and waist circumference than found in other ethnic groups.36 Obesity, especially upper body obesity, is associated with insulin resistance, higher triglycerides, and lower HDL37 in HIV-infected patients.
The contribution of illicit drug use and anabolic steroids to fat distribution is not understood and difficult to interpret in the clinical context. As a wider variety of patients undergo study and treatment, it must be recognized that genetic and environmental factors38 may add to the superimposed HIV-and antiretroviral drug–induced changes now occurring in the era of HAART.
Renal Disease
Renal disease is associated with increased CVD. In the era before HAART, the major renal disease was HIV-associated nephropathy characterized by proteinuria.39 HIV-associated nephropathy leads to end-stage renal disease requiring dialysis.39 End-stage renal disease currently is the fourth-leading cause of death in HIV infection.40 The prevalence of proteinuria ranged from 19% to 34% in the pre-HAART era. Risk factors include black race, high HIV RNA, and low CD4 count.41
The Effect of HAART and Specific Antiretrovirals
In evaluating the evidence available, we must consider 2 factors. First, early studies were conducted in HIV-infected patients receiving ineffective single or dual therapies. Second, it is difficult to distinguish the separate contributions of individual drugs and drug classes in HAART. Some changes may be induced by only some drugs in a class, whereas other changes may be induced by all drugs in the class. In addition to randomized trials, informative data come from studies of antiretroviral administration to HIV-negative healthy volunteers.
Lipids and Lipoproteins
Historically, the sentinel signal of disturbed lipid metabolism caused by antiretroviral therapy itself was the paradoxical change in triglycerides. Whereas azidothymidine therapy decreased the elevated triglycerides found in advanced HIV infection42 (the Table), the addition of another drug class, the protease inhibitors (PIs), increased triglycerides.43–45 PI drugs also increased LDL-C with little or no change in HDL-C.45 These changes in lipid metabolism occur before any observable change in body composition.45
These changes may occur less often with the newer drugs now available to treat HIV.46 In the current era, when patients are healthier at the time of HIV diagnosis, it also must be recognized that dyslipidemias are common in the general population and are due to genetic and environmental causes (eg, diet, obesity, sedentary lifestyle). Preexisting dyslipidemia may be exacerbated by HIV treatment. Those with underlying genetic predisposition may have worse lipids during antiretroviral treatment.47
Factors Associated With Increases in Triglycerides
Ritonavir-based PI regimens induce the greatest increase in triglycerides in a dose-dependent manner.43,48–51 As studies in HIV-infected and HIV-negative subjects show, most other PIs have little effect.51,52 Efavirenz and stavudine also may increase triglycerides in patients with HIV infection.53–55 Severe lipoatrophy (see below), increased VAT, and increased upper trunk fat are associated with elevated triglycerides.56,57
Factors Associated With Increases in LDL-C
The increase in LDL-C with HAART is modest because it represents mainly the consequence of effective treatment of HIV, reversing the small decrease in LDL-C caused by HIV45,48,58 (the Table). Most HAART regimens, whether they contain PI or non-NRTI (NNRTI) drugs, raise LDL-C, except perhaps for some atazanavir-based regimens.46,55,58,59 LDL-C elevations after HAART may be due to modulation of the immune response to HIV, restoration of health, or both. These changes are unlikely to move an individual from low or moderate CVD risk to a high-risk category. Significantly elevated LDL in the context of HIV is likely due to other causes, including genetics and diets high in saturated and trans fats (which may be more common in the HIV-infected population).60
Factors Associated With Decreased HDL
The ability of PIs to increase HDL is modest at best (the Table).45,48,58 Effects of NNRTIs and perhaps atazanavir in increasing HDL are more impressive.46,55,58,59 Increased VAT and upper trunk fat are associated with low HDL. Nevirapine induces the largest increase.53 The persisting decreased levels of HDL-C may be a key mechanism by which HIV promotes CVD in the era of HAART.
Glucose Metabolism and Insulin Resistance
PI-induced increases in glucose and insulin resistance occur before observable changes in body composition (the Table).45,52,61 Newer PI drugs appear to have less effect.62–64 Specific NRTIs, particularly thymidine analogues, also may contribute to insulin resistance by an independent effect possibly related to mitochondrial dysfunction.18,65,66 Insulin resistance in HIV-infected patients also occurs late after initiation of HAART therapy, which may not be a direct drug toxicity.67
Factors Associated With Increased Insulin Resistance
Direct effects of PI drugs have been demonstrated in HIV-negative volunteers. Indinavir induces the greatest insulin resistance, followed by ritonavir, with little effect of other PIs.50,52,61,63,64,68,69 Some data support the induction of insulin resistance by NRTI, particularly stavudine and zidovudine.18,65,66 Insulin resistance increases with time after initial drug effects and occurs on most regimens; restoration to health or subsequent changes in body fat composition may mediate this phenomenon.67 Increased VAT and trunk fat are associated with insulin resistance; lipoatrophy also may contribute in HIV-infected patients.37,70
Factors Associated With Increased Prevalence of IGT and Diabetes
Although an increased prevalence of diabetes and, to a lesser extent, impaired glucose tolerance has been found in HIV infection, the studies of the associated causes are less consistent or have not been repeated. Early reports invoked the PI indinavir as a cause of diabetes, but more recently, diabetes has been associated with use of ritonavir, NRTIs, especially stavudine, and HAART itself.17,71–75 Some studies link PIs to defects in insulin secretion, a necessary step in the development of diabetes.74,76–78 Ethnic and genetic predisposition plays a role.
Body Composition
Lipoatrophy is an HIV-specific change that occurs with certain antiretrovirals (the Table). Lipoatrophy affects all subcutaneous adipose tissue depots, with the least loss of fat in the upper trunk.54,79–83 The major culprits in lipoatrophy are thymidine analogue NRTI drugs (especially stavudine).30,65,80,81,84–89 The effects of NNRTIs and PIs are less clear, if not controversial: Some PIs may worsen lipoatrophy (eg, indinavir), others may not, whereas there may be an effect of some NNRTIs (eg, efavirenz).80,81,85,88,90 The relative sparing of upper trunk fat likely contributes to the appearance of “buffalo hump.”
Lipohypertrophy refers to increases in adipose tissue, in particular depots including VAT and upper trunk, especially in the breasts and dorsocervical fat pads. High levels of VAT may occur in HIV infection despite subcutaneous lipoatrophy.79–83 The presence of lipohypertrophy is not linked to or the result of lipoatrophy in other depots. Indeed, most evidence indicates that lipohypertrophy is disassociated from lipoatrophy. The drug factors that contribute to lipoatrophy are not associated with lipohypertrophy. Effective viral suppression, restoration to health, and weight gain play significant roles in lipohypertrophy.79–83
Increased VAT is associated with increased inflammation even in the absence of HIV.21,91 HIV infection itself is associated with inflammation. It is not known whether inflammation from HIV infection leads to increased VAT. Other evidence suggests that HIV lipoatrophy is associated with inflammation in adipose tissue.92
Terminology Does Not Describe What Is Happening in Adipose Tissue
Lipohypertrophy is a term that refers to a measurable increase in fat volume. However, it is not known whether at a cellular level this represents adipocyte hypertrophy (increase in cell size) or hyperplasia (increase in cell number). Some data suggest that fat cell size, not merely fat mass, predicts a worse metabolic profile.93–95
Lipoatrophy is a term that refers to a measurable decrease in fat volume. Again, it is not known whether this is due to smaller fat cells or fewer fat cells (eg, apoptosis). There is evidence that lipoatrophy is accompanied by abnormalities of fat cell regulation that do not occur with decreased fat mass as a result of negative caloric balance.92,96 Among non–HIV-infected patients, evidence suggests potentially significant genetic contributions to fat loss in lipodystrophy syndromes. Less is known regarding the genetic contributions to fat loss in HIV-infected patients and whether genetic influences, viral factors, inflammatory mediators, and antiretroviral effects may interact to contribute to lipoatrophy in HIV-infected patients. Studies to determine potential genetic predictors of lipoatrophy in HIV-infected patients are ongoing.
Metabolic Syndrome
A number of the changes seen with HIV infection, restoration to health, and treatment with HAART, including dyslipidemia, diabetes, and increased BMI and waist circumference, may present simultaneously in HIV-infected patients. These factors are part of the metabolic syndrome.97,98 There is debate as to whether the prevalence of the metabolic syndrome is increased among HIV-infected patients.99 Furthermore, it remains unknown whether the presence of the metabolic syndrome per se confers increased risk for CVD disease in HIV-infected patients beyond that associated with individual risk factors.99,100
Renal Disease
Treatment with HAART has led to a reduction in HIV-associated nephropathy, but renal disease remains prominent in HIV-infected people. Microalbuminuria, a marker of both renal and CVD, is 5-fold more common in HIV infection even after adjustment for known factors associated with microalbuminuria.101 Although microalbuminuria is still associated with low CD4 count, high HIV RNA, and black race, in the era of HAART, microalbuminuria also is associated with potential CVD risk factors, including age, blood pressure, insulin resistance, glycosuria, and triglyceride levels.
Cystatin C is a measure of glomerular function that, unlike creatinine-based estimates of glomerular filtration (including equations for estimated glomerular filtration rate), is not affected by body composition. Elevated cystatin C levels predict renal failure but, in addition, strongly predict CVD and all-cause mortality in the general population. People with HIV infection are 9.8-fold more likely to have elevated cystatin C levels, even after adjustment for the known associated factors.102 Elevated cystatin C levels are associated with traditional risk factors of hypertension, low HDL, uric acid levels, albuminuria, or proteinuria but not with impaired fasting glucose or diabetes. Elevated cystatin C levels were strongly associated with low CD4 count and coinfection with hepatitis C. There is debate over the extent to which tenofovir use leads to decreased renal function as measured by creatinine, creatinine clearance, and cystatin C levels.103–107 The implications of these findings for end-stage renal disease and CVD are not yet known.
Controversial Issues, Gaps in Knowledge, and Future Research Priorities
To understand how HIV infection and the host response to it affects metabolism and body composition, patients with newly diagnosed HIV infection need to be studied with measurements of lipids, lipoproteins, glucose metabolism, insulin resistance, body composition, and renal function in the window before HAART is required. Research is needed into the aspects of the host response to infection that cause these changes.
To understand how HAART affects metabolism, body composition, and renal function, the effects of new and emerging antiretroviral drugs and HAART regimens need to be studied with measurements of lipids, lipoproteins, glucose metabolism, insulin resistance, body composition, and renal function.
What are the contributions of mitochondrial toxicity/mitochondrial dysfunction, intramyocellular fat, lean body mass, fatty liver, and hepatitis C virus coinfection to metabolic and body fat distribution?
How should obesity be assessed in patients with HIV-associated lipoatrophy, and what are the implications of obesity for cardiovascular risk in HIV infection?
What are the contributions of antiretroviral-induced diabetes to CVD?
Do markers of renal disease predict CVD and mortality in HIV infection similar to their predictive ability in noninfected populations?
Those studied should include all groups in the current HIV epidemic (women, blacks, Asians, and the elderly) because there are indications of differences in response to HIV infection and HAART.
Longer-term research should assess the contribution of these HIV- and HAART-induced changes to atherosclerosis.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
The opinions expressed in this manuscript are those of the authors and should not be construed as necessarily representing an official position of the US Department of Health and Human Services, the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, or the US government. These opinions are not necessarily those of the editor or the American Heart Association.
Disclosures
Potential conflicts of interest for members of the writing groups for all sections of these conference proceedings are provided in a disclosure Table included with the Executive Summary, which is available online at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.189622.
References
- 1.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 2.Constans J, Pellegrin JL, Peuchant E, Dumon MF, Pellegrin I, Sergeant C, Simonoff M, Brossard G, Barbeau P, Fleury H, Clerc M, Leng B, Conri C. Plasma lipids in HIV-infected patients: a prospective study in 95 patients. Eur J Clin Invest. 1994;24:416–420. doi: 10.1111/j.1365-2362.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 3.El-Sadr WM, Mullin CM, Carr A, Gibert C, Rappoport C, Visnegarwala F, Grunfeld C, Raghavan SS. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005;6:114–121. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 4.Grunfeld C, Kotler DP, Shigenaga JK, Doerrler W, Tierney A, Wang J, Pierson RN, Jr, Feingold KR. Circulating interferon-alpha levels and hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1991;90:154–162. [PubMed] [Google Scholar]
- 5.Hellerstein MK, Grunfeld C, Wu K, Christiansen M, Kaempfer S, Kletke C, Shackleton CH. Increased de novo hepatic lipogenesis in human immunodeficiency virus infection. J Clin Endocrinol Metab. 1993;76:559–565. doi: 10.1210/jcem.76.3.8445011. [DOI] [PubMed] [Google Scholar]
- 6.Feingold K, Krauss RM, Pang M, Doerrler W, Jensen P, Grunfeld C. The hypertriglyceridemia of acquired immunodeficiency syndrome is associated with an increased prevalence of low density lipoprotein subclass pattern B. J Clin Endocrinol Metab. 1993;76:1423–1427. doi: 10.1210/jcem.76.6.8501146. [DOI] [PubMed] [Google Scholar]
- 7.Chait A, Brazg RL, Tribble DL, Krauss RM. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am J Med. 1993;94:350–356. doi: 10.1016/0002-9343(93)90144-e. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 9.Duong M, Petit JM, Martha B, Galland F, Piroth L, Walldner A, Grappin M, Buisson M, Duvillard L, Chavanet P, Portier H. Concentration of circulating oxidized LDL in HIV-infected patients treated with antiretroviral agents: relation to HIV-related lipodystrophy. HIV Clin Trials. 2006;7:41–47. doi: 10.1310/7381-m1yd-rtv5-4ryt. [DOI] [PubMed] [Google Scholar]
- 10.Neuenburg JK, Furlan S, Bacchetti P, Price RW, Grant RM. Enrichment of activated monocytes in cerebrospinal fluid during antiretroviral therapy. AIDS. 2005;19:1351–1359. doi: 10.1097/01.aids.0000181008.39514.ee. [DOI] [PubMed] [Google Scholar]
- 11.Khovidhunkit W, Memon RA, Shigenaga JK, Pang M, Schambelan M, Mulligan K, Feingold KR, Grunfeld C. Plasma platelet-activating factor acetylhydrolase activity in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Metabolism. 1999;48:1524–1531. doi: 10.1016/s0026-0495(99)90240-8. [DOI] [PubMed] [Google Scholar]
- 12.Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, Fu Y, Dart A, Orenstein JM, Bobryshev YV, Bukrinsky M, Sviridov D. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;4:e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–710. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 14.Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD for the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1046/j.1468-1293.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 15.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 16.Hommes MJ, Romijn JA, Endert E, Eeftinck Schattenkerk JK, Sauerwein HP. Insulin sensitivity and insulin clearance in human immunodeficiency virus-infected men. Metabolism. 1991;40:651–656. doi: 10.1016/0026-0495(91)90059-6. [DOI] [PubMed] [Google Scholar]
- 17.Walli R, Herfort O, Michl GM, Demant T, Jäger H, Dieterle C, Bogner JR, Landgraf R, Goebel FD. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–F173. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Brown TT, Li X, Cole SR, Kingsley LA, Palella FJ, Riddler SA, Chmiel JS, Visscher BR, Margolick JB, Dobs AS. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS. 2005;19:1375–1383. doi: 10.1097/01.aids.0000181011.62385.91. [DOI] [PubMed] [Google Scholar]
- 19.Kotler DP, Wang J, Pierson RN. Body composition studies in patients with the acquired immunodeficiency syndrome. Am J Clin Nutr. 1985;42:1255–1265. doi: 10.1093/ajcn/42.6.1255. [DOI] [PubMed] [Google Scholar]
- 20.Kotler DP, Rosenbaum K, Wang J, Pierson RN. Studies of body composition and fat distribution in HIV-infected and control subjects. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:228–237. doi: 10.1097/00042560-199903010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Despres JP. Health consequences of visceral obesity. Ann Med. 2001;33:534–541. doi: 10.3109/07853890108995963. [DOI] [PubMed] [Google Scholar]
- 22.Grunfeld C, Feingold KR. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- 23.Grunfeld C, Pang M, Shimizu L, Shigenaga JK, Jensen P, Feingold KR. Resting energy expenditure, caloric intake, and short-term weight change in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Am J Clin Nutr. 1992;55:455–460. doi: 10.1093/ajcn/55.2.455. [DOI] [PubMed] [Google Scholar]
- 24.Macallan DC, Noble C, Baldwin C, Foskett M, McManus T, Griffin GE. Prospective analysis of patterns of weight change in stage IV human immunodeficiency virus infection. Am J Clin Nutr. 1993;58:417–424. doi: 10.1093/ajcn/58.3.417. [DOI] [PubMed] [Google Scholar]
- 25.Macallan DC, Noble C, Baldwin C, Jebb SA, Prentice AM, Coward WA, Sawyer MB, McManus TJ, Griffin GE. Energy expenditure and wasting in human immunodeficiency virus infection. N Engl J Med. 1995;333:83–88. doi: 10.1056/NEJM199507133330202. [DOI] [PubMed] [Google Scholar]
- 26.Suttmann U, Ockenga J, Selberg O, Hoogestraat L, Deicher H, Muller MJ. Incidence and prognostic value of malnutrition and wasting in human immunodeficiency virus-infected outpatients. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:239–246. doi: 10.1097/00042560-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ott M, Fischer H, Polat H, Helm EB, Frenz M, Caspary WF, Lembcke B. Bioelectrical impedance analysis as a predictor of survival in patients with human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:20–25. [PubMed] [Google Scholar]
- 28.Sharkey SJ, Sharkey KA, Sutherland LR, Church DL. Nutritional status and food intake in human immunodeficiency virus infection: GI/HIV Study Group. J Acquir Immune Defic Syndr. 1992;5:1091–1098. [PubMed] [Google Scholar]
- 29.Mulligan K, Tai VW, Schambelan M. Cross-sectional and longitudinal evaluation of body composition in men with HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:43–48. doi: 10.1097/00042560-199705010-00007. [DOI] [PubMed] [Google Scholar]
- 30.Saint-Marc T, Partisani M, Poizot-Martin I, Bruno F, Rouviere O, Lang JM, Gastaut JA, Touraine JL. A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. AIDS. 1999;13:1659–1667. doi: 10.1097/00002030-199909100-00009. [DOI] [PubMed] [Google Scholar]
- 31.Ogiwara H, Takahashi S, Kato Y, Uyama I, Takahara T, Kikuchi K, Iida S. Diminished visceral adipose tissue in cancer cachexia. J Surg Oncol. 1994;57:129–133. doi: 10.1002/jso.2930570211. [DOI] [PubMed] [Google Scholar]
- 32.Zamboni M, Armellini F, Turcato E, Todisco P, Gallagher D, Dalle Grave R, Heymsfield S, Bosello O. Body fat distribution before and after weight gain in anorexia nervosa. Int J Obes Relat Metab Disord. 1997;21:33–36. doi: 10.1038/sj.ijo.0800357. [DOI] [PubMed] [Google Scholar]
- 33.Fujimoto WY, Jablonski KA, Bray GA, Kriska A, Barrett-Connor E, Haffner S, Hanson R, Hill JO, Hubbard V, Stamm E, Pi-Sunyer FX for the Diabetes Prevention Program Research Group. Body size and shape changes and the risk of diabetes in the Diabetes Prevention Program. Diabetes. 2007;56:1680–1685. doi: 10.2337/db07-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sidney S, Lewis CE, Hill JO, Quesenberry CP, Jr, Stamm ER, Scherzinger A, Tolan K, Ettinger B. Association of total and central adiposity measures with fasting insulin in a biracial population of young adults with normal glucose tolerance: the CARDIA study. Obes Res. 1999;7:265–272. doi: 10.1002/j.1550-8528.1999.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 35.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–387. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 36.Fujimoto WY, Abbate SL, Kahn SE, Hokanson JE, Brunzell JD. The visceral adiposity syndrome in Japanese-American men. Obes Res. 1994;2:364–371. doi: 10.1002/j.1550-8528.1994.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 37.Grunfeld C, Rimland D, Gibert CL, Powderly WG, Sidney S, Shlipak MG, Bacchetti P, Scherzer R, Haffner S, Heymsfield SB. Association of upper trunk and visceral adipose tissue with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr. 2007;46:283–290. doi: 10.1097/qai.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouchard C, Despres JP, Mauriege P. Genetic and nongenetic determinants of regional fat distribution. Endocr Rev. 1993;14:72–93. doi: 10.1210/edrv-14-1-72. [DOI] [PubMed] [Google Scholar]
- 39.Szczech LA, Gange SJ, van der Horst C, Bartlett JA, Young M, Cohen MH, Anastos K, Klassen PS, Svetkey LP. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 40.Selik RM, Byers RH, Jr, Dworkin MS. Trends in diseases reported on U.S. death certificates that mentioned HIV infection, 1987–1999. J Acquir Immune Defic Syndr. 2002;29:378–387. doi: 10.1097/00126334-200204010-00009. [DOI] [PubMed] [Google Scholar]
- 41.Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, Fields TA, Svetkey LP, Flanagan KH, Klotman PE, Winston JA. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 42.Mildvan D, Machado SG, Wilets I, Grossberg SE. Endogenous interferon and triglyceride concentrations to assess response to zidovudine in AIDS and advanced AIDS-related complex. Lancet. 1992;339:453–456. doi: 10.1016/0140-6736(92)91058-g. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan AK, Nelson MR. Marked hyperlipidaemia on ritonavir therapy. AIDS. 1997;11:938–939. [PubMed] [Google Scholar]
- 44.Dong KL, Bausserman LL, Flynn MM, Dickinson BP, Flanigan TP, Mileno MD, Tashima KT, Carpenter CC. Changes in body habitus and serum lipid abnormalities in HIV-positive women on highly active antiretroviral therapy (HAART) J Acquir Immune Defic Syndr. 1999;21:107–113. [PubMed] [Google Scholar]
- 45.Mulligan K, Grunfeld C, Tai VW, Algren H, Pang M, Chernoff DN, Lo JC, Schambelan M. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr. 2000;23:35–43. doi: 10.1097/00126334-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 46.Murphy RL, Sanne I, Cahn P, Phanuphak P, Percival L, Kelleher T, Giordano M. Dose-ranging, randomized, clinical trial of atazanavir with lamivudine and stavudine in antiretroviral-naive subjects: 48-week results. AIDS. 2003;17:2603–2614. doi: 10.1097/00002030-200312050-00007. [DOI] [PubMed] [Google Scholar]
- 47.Behrens G, Schmidt HH, Stoll M, Schmidt RE. ApoE genotype and protease-inhibitor-associated hyperlipidaemia. Lancet. 1999;354:76. doi: 10.1016/S0140-6736(05)75347-2. [DOI] [PubMed] [Google Scholar]
- 48.Periard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, Marcovina SM, Glauser MP, Nicod P, Darioli R, Mooser V. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors: the Swiss HIV Cohort Study. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 49.Purnell JQ, Zambon A, Knopp RH, Pizzuti DJ, Achari R, Leonard JM, Locke C, Brunzell JD. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS. 2000;14:51–57. doi: 10.1097/00002030-200001070-00006. [DOI] [PubMed] [Google Scholar]
- 50.Lee GA, Seneviratne T, Noor MA, Lo JC, Schwarz JM, Aweeka FT, Mulligan K, Schambelan M, Grunfeld C. The metabolic effects of lopinavir/ritonavir in HIV-negative men. AIDS. 2004;18:641–649. doi: 10.1097/00002030-200403050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadler BM, Piliero PJ, Preston SL, Lloyd PP, Lou Y, Stein DS. Pharmacokinetics and safety of amprenavir and ritonavir following multiple-dose, co-administration to healthy volunteers. AIDS. 2001;15:1009–1018. doi: 10.1097/00002030-200105250-00009. [DOI] [PubMed] [Google Scholar]
- 52.Noor MA, Lo JC, Mulligan K, Schwarz JM, Halvorsen RA, Schambelan M, Grunfeld C. Metabolic effects of indinavir in healthy HIV-seronegative men. AIDS. 2001;15:F11–F18. doi: 10.1097/00002030-200105040-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Leth F, Phanuphak P, Stroes E, Gazzard B, Cahn P, Raffi F, Wood R, Bloch M, Katlama C, Kastelein JJ, Schechter M, Murphy RL, Horban A, Hall DB, Lange JM, Reiss P. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med. 2004;1:e19. doi: 10.1371/journal.pmed.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 55.Fontas E, van Leth F, Sabin CA, Friis-Moller N, Rickenbach M, d’Arminio Monforte A, Kirk O, Dupon M, Morfeldt L, Mateu S, Petoumenos K, El-Sadr W, de Wit S, Lundgren JD, Pradier C, Reiss P for the D:A:D: Study Group. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis. 2004;189:1056–1074. doi: 10.1086/381783. [DOI] [PubMed] [Google Scholar]
- 56.Currier J, Scherzer R, Bacchetti P, Heymsfield S, Lee D, Sidney S, Tien PC for the Fat Redistribution and Metabolic Changes in HIV Infection Study Investigators. Regional adipose tissue and lipid and lipoprotein levels in HIV-infected women. J Acquir Immune Defic Syndr. 2008;48:35–43. doi: 10.1097/QAI.0b013e318164227f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wohl D, Scherzer R, Heymsfield S, Simberkoff M, Sidney S, Bacchetti P, Grunfeld C for the FRAM Study Investigators. The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr. 2008;48:44–52. doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Valk M, Kastelein JJ, Murphy RL, van Leth F, Katlama C, Horban A, Glesby M, Behrens G, Clotet B, Stellato RK, Molhuizen HO, Reiss P for the Atlantic Study Team. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15:2407–2414. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 59.Fisac C, Virgili N, Ferrer E, Barbera MJ, Fumero E, Vilarasau C, Podzamczer D. A comparison of the effects of nevirapine and nelfinavir on metabolism and body habitus in antiretroviral-naive human immunodeficiency virus-infected patients: a randomized controlled study. J Clin Endocrinol Metab. 2003;88:5186–5192. doi: 10.1210/jc.2002-021830. [DOI] [PubMed] [Google Scholar]
- 60.Joy T, Keogh HM, Hadigan C, Lee H, Dolan SE, Fitch K, Liebau J, Lo J, Johnsen S, Hubbard J, Anderson EJ, Grinspoon S. Dietary fat intake and relationship to serum lipid levels in HIV-infected patients with metabolic abnormalities in the HAART era. AIDS. 2007;21:1591–1600. doi: 10.1097/QAD.0b013e32823644ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noor MA, Seneviratne T, Aweeka FT, Lo JC, Schwarz JM, Mulligan K, Schambelan M, Grunfeld C. Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: a randomized, placebo-controlled study. AIDS. 2002;16:F1–F8. doi: 10.1097/00002030-200203290-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dube MP, Qian D, Edmondson-Melancon H, Sattler FR, Goodwin D, Martinez C, Williams V, Johnson D, Buchanan TA. Prospective, intensive study of metabolic changes associated with 48 weeks of amprenavir-based antiretroviral therapy. Clin Infect Dis. 2002;35:475–481. doi: 10.1086/341489. [DOI] [PubMed] [Google Scholar]
- 63.Noor MA, Flint OP, Maa JF, Parker RA. Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose uptake and insulin sensitivity: demonstrable differences in vitro and clinically. AIDS. 2006;20:1813–1821. doi: 10.1097/01.aids.0000244200.11006.55. [DOI] [PubMed] [Google Scholar]
- 64.Noor MA, Parker RA, O’Mara E, Grasela DM, Currie A, Hodder SL, Fiedorek FT, Haas DW. The effects of HIV protease inhibitors atazanavir and lopinavir/ritonavir on insulin sensitivity in HIV-seronegative healthy adults. AIDS. 2004;18:2137–2144. doi: 10.1097/00002030-200411050-00005. [DOI] [PubMed] [Google Scholar]
- 65.Shlay JC, Visnegarwala F, Bartsch G, Wang J, Peng G, El-Sadr WM, Gibert C, Kotler D, Grunfeld C, Raghavan S for the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) Body composition and metabolic changes in antiretroviral-naive patients randomized to didanosine and stavudine vs. abacavir and lamivudine. J Acquir Immune Defic Syndr. 2005;38:147–155. doi: 10.1097/01.qai.0000143599.64234.15. [DOI] [PubMed] [Google Scholar]
- 66.Fleischman A, Johnsen S, Systrom DM, Hrovat M, Farrar CT, Frontera WR, Fitch K, Thomas BJ, Torriani M, Cote HC, Grinspoon SK. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol Endocrinol Metab. 2007;292:E1666–E1673. doi: 10.1152/ajpendo.00550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shlay JC, Bartsch G, Peng G, Wang J, Grunfeld C, Gibert CL, Visnegarwala F, Raghavan SS, Xiang Y, Farrough M, Perry HE, Kotler D, El-Sadr WM. Long-term body composition and metabolic changes in antiretroviral naive persons randomized to protease inhibitor-, non-nucleoside reverse transcriptase inhibitor-, or protease inhibitor plus nonnucleoside reverse transcriptase inhibitor-based strategy. J Acquir Immune Defic Syndr. 2007;44:506–517. doi: 10.1097/QAI.0b013e31804216cf. [DOI] [PubMed] [Google Scholar]
- 68.Lee GA, Lo JC, Aweeka F, Schwarz JM, Mulligan K, Schambelan M, Grunfeld C. Single-dose lopinavir-ritonavir acutely inhibits insulin-mediated glucose disposal in healthy volunteers. Clin Infect Dis. 2006;43:658–660. doi: 10.1086/505974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee GA, Rao MN, Mulligan K, Lo JC, Aweeka F, Schwarz JM, Schambelan M, Grunfeld C. Effects of ritonavir and amprenavir on insulin sensitivity in healthy volunteers. AIDS. 2007;21:2183–2190. doi: 10.1097/QAD.0b013e32826fbc54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mynarcik DC, McNurlan MA, Steigbigel RT, Fuhrer J, Gelato MC. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr. 2000;25:312–321. doi: 10.1097/00042560-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 71.Visnegarwala F, Krause KL, Musher DM. Severe diabetes associated with protease inhibitor therapy. Ann Intern Med. 1997;127:947. doi: 10.7326/0003-4819-127-10-199711150-00016. [DOI] [PubMed] [Google Scholar]
- 72.Dube MP, Johnson DL, Currier JS, Leedom JM. Protease inhibitor-associated hyperglycaemia. Lancet. 1997;350:713–714. doi: 10.1016/S0140-6736(05)63513-1. [DOI] [PubMed] [Google Scholar]
- 73.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the Multicenter AIDS Cohort Study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 74.Behrens G, Dejam A, Schmidt H, Balks HJ, Brabant G, Korner T, Stoll M, Schmidt RE. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–F70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 75.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 76.Woerle HJ, Mariuz PR, Meyer C, Reichman RC, Popa EM, Dostou JM, Welle SL, Gerich JE. Mechanisms for the deterioration in glucose tolerance associated with HIV protease inhibitor regimens. Diabetes. 2003;52:918–925. doi: 10.2337/diabetes.52.4.918. [DOI] [PubMed] [Google Scholar]
- 77.Koster JC, Remedi MS, Qiu H, Nichols CG, Hruz PW. HIV protease inhibitors acutely impair glucose-stimulated insulin release. Diabetes. 2003;52:1695–1700. doi: 10.2337/diabetes.52.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dufer M, Neye Y, Krippeit-Drews P, Drews G. Direct interference of HIV protease inhibitors with pancreatic beta-cell function. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:583–590. doi: 10.1007/s00210-004-0933-6. [DOI] [PubMed] [Google Scholar]
- 79.Tien PC, Cole SR, Williams CM, Li R, Justman JE, Cohen MH, Young M, Rubin N, Augenbraun M, Grunfeld C. Incidence of lipoatrophy and lipohypertrophy in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2003;34:461–466. doi: 10.1097/00126334-200312150-00003. [DOI] [PubMed] [Google Scholar]
- 80.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, Saag M, Scherzer R, Shlipak M, Tien P for the Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562–571. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palella FJ, Jr, Cole SR, Chmiel JS, Riddler SA, Visscher B, Dobs A, Williams C. Anthropometrics and examiner-reported body habitus abnormalities in the Multicenter AIDS Cohort Study. Clin Infect Dis. 2004;38:903–907. doi: 10.1086/381684. [DOI] [PubMed] [Google Scholar]
- 83.Mulligan K, Parker RA, Komarow L, Grinspoon SK, Tebas P, Robbins GK, Roubenoff R, Dube MP. Mixed patterns of changes in central and peripheral fat following initiation of antiretroviral therapy in a randomized trial. J Acquir Immune Defic Syndr. 2006;41:590–597. doi: 10.1097/01.qai.0000214811.72916.67. [DOI] [PubMed] [Google Scholar]
- 84.Saint-Marc T, Partisani M, Poizot-Martin I, Rouviere O, Bruno F, Avellaneda R, Lang JM, Gastaut JA, Touraine JL. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: preliminary results of the LIPOCO study. AIDS. 2000;14:37–49. doi: 10.1097/00002030-200001070-00005. [DOI] [PubMed] [Google Scholar]
- 85.Mallal SA, John M, Moore CB, James IR, McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS. 2000;14:1309–1316. doi: 10.1097/00002030-200007070-00002. [DOI] [PubMed] [Google Scholar]
- 86.Bogner JR, Vielhauer V, Beckmann RA, Michl G, Wille L, Salzberger B, Goebel FD. Stavudine versus zidovudine and the development of lipodystrophy. J Acquir Immune Defic Syndr. 2001;27:237–244. doi: 10.1097/00126334-200107010-00004. [DOI] [PubMed] [Google Scholar]
- 87.Bernasconi E, Boubaker K, Junghans C, Flepp M, Furrer HJ, Haensel A, Hirschel B, Boggian K, Chave JP, Opravil M, Weber R, Rickenbach M, Telenti A for the Swiss HIV Cohort Study. Abnormalities of body fat distribution in HIV-infected persons treated with antiretroviral drugs: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2002;31:50–55. doi: 10.1097/00126334-200209010-00007. [DOI] [PubMed] [Google Scholar]
- 88.Dube MP, Parker RA, Tebas P, Grinspoon SK, Zackin RA, Robbins GK, Roubenoff R, Shafer RW, Wininger DA, Meyer WA, 3rd, Snyder SW, Mulligan K. Glucose metabolism, lipid, and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS. 2005;19:1807–1818. doi: 10.1097/01.aids.0000183629.20041.bb. [DOI] [PubMed] [Google Scholar]
- 89.Podzamczer D, Ferrer E, Sanchez P, Gatell JM, Crespo M, Fisac C, Lonca M, Sanz J, Niubo J, Veloso S, Llibre JM, Barrufet P, Ribas MA, Merino E, Ribera E, Martinez-Lacasa J, Alonso C, Aranda M, Pulido F, Berenguer J, Delegido A, Pedreira JD, Lerida A, Rubio R, del Rio L for the ABDCE (Abacavir vs. d4T (stavudine) Plus Efavirenz) Study Team. Less lipoatrophy and better lipid profile with abacavir as compared to stavudine: 96-week results of a randomized study. J Acquir Immune Defic Syndr. 2007;44:139–147. doi: 10.1097/QAI.0b013e31802bf122. [DOI] [PubMed] [Google Scholar]
- 90.Dube MP, Komarow L, Mulligan K, Grinspoon SK, Parker RA, Robbins GK, Roubenoff R, Tebas P for the Adult Clinical Trials Group 384. Long-term body fat outcomes in antiretroviral-naive participants randomized to nelfinavir or efavirenz or both plus dual nucleosides: dual X-ray absorptiometry results from A5005s, a substudy of Adult Clinical Trials Group 384. J Acquir Immune Defic Syndr. 2007;45:508–514. doi: 10.1097/QAI.0b013e3181142d26. [DOI] [PubMed] [Google Scholar]
- 91.Forouhi NG, Sattar N, McKeigue PM. Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. Int J Obes Relat Metab Disord. 2001;25:1327–1331. doi: 10.1038/sj.ijo.0801723. [DOI] [PubMed] [Google Scholar]
- 92.Jan V, Cervera P, Maachi M, Baudrimont M, Kim M, Vidal H, Girard PM, Levan P, Rozenbaum W, Lombes A, Capeau J, Bastard JP. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir Ther. 2004;9:555–564. [PubMed] [Google Scholar]
- 93.Karam JH. Obesity: fat cells–not fat people. West J Med. 1979;130:128–132. [PMC free article] [PubMed] [Google Scholar]
- 94.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 95.Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and “hyperleptinaemia”. Diabetologia. 2007;50:625–633. doi: 10.1007/s00125-006-0572-1. [DOI] [PubMed] [Google Scholar]
- 96.Kosmiski LA, Bacchetti P, Kotler DP, Heymsfield SB, Lewis CE, Shlipak MG, Scherzer R, Grunfeld C. Relationship of fat distribution with adipokines in human immunodeficiency virus infection. J Clin Endocrinol Metab. 2007;93:216–224. doi: 10.1210/jc.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 98.Balkau B, Charles MA, Drivsholm T, Borch-Johnsen K, Wareham N, Yudkin JS, Morris R, Zavaroni I, van Dam R, Feskins E, Gabriel R, Diet M, Nilsson P, Hedblad B for the European Group for the Study of Insulin Resistance (EGIR) Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28:364–376. [PubMed] [Google Scholar]
- 99.Pao V, Lee GA, Grunfeld C. HIV therapy, metabolic syndrome, and cardiovascular risk. Curr Atheroscler Rep. 2008;10:61–70. doi: 10.1007/s11883-008-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kahn R, Buse J, Ferrannini E, Stern M for the American Diabetes Association, European Association for the Study of Diabetes. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 101.Szczech LA, Grunfeld C, Scherzer R, Canchola JA, van der Horst C, Sidney S, Wohl D, Shlipak MG. Microalbuminuria in HIV infection. AIDS. 2007;21:1003–1009. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Odden M, Scherzer R, Bacchetti P, Szczech LA, Sidney S, Grunfeld C, Shlipak M. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007;167:2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mauss S, Berger F, Schmutz G. Antiretroviral therapy with tenofovir is associated with mild renal dysfunction. AIDS. 2005;19:93–95. doi: 10.1097/00002030-200501030-00012. [DOI] [PubMed] [Google Scholar]
- 104.Antoniou T, Raboud J, Chirhin S, Yoong D, Govan V, Gough K, Rachlis A, Loutfy M. Incidence of and risk factors for tenofovir-induced nephrotoxicity: a retrospective cohort study. HIV Med. 2005;6:284–290. doi: 10.1111/j.1468-1293.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 105.Jones R, Stebbing J, Nelson M, Moyle G, Bower M, Mandalia S, Gazzard B. Renal dysfunction with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy regimens is not observed more frequently: a cohort and case-control study. J Acquir Immune Defic Syndr. 2004;37:1489–1495. doi: 10.1097/01.qai.0000138983.45235.02. [DOI] [PubMed] [Google Scholar]
- 106.Izzedine H, Hulot JS, Vittecoq D, Gallant JE, Staszewski S, Launay-Vacher V, Cheng A, Deray G for the Study 903 Team. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients: data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant. 2005;20:743–746. doi: 10.1093/ndt/gfh658. [DOI] [PubMed] [Google Scholar]
- 107.Fux CA, Christen A, Zgraggen S, Mohaupt MG, Furrer H. Effect of tenofovir on renal glomerular and tubular function. AIDS. 2007;21:1483–1485. doi: 10.1097/QAD.0b013e328216f15b. [DOI] [PubMed] [Google Scholar]