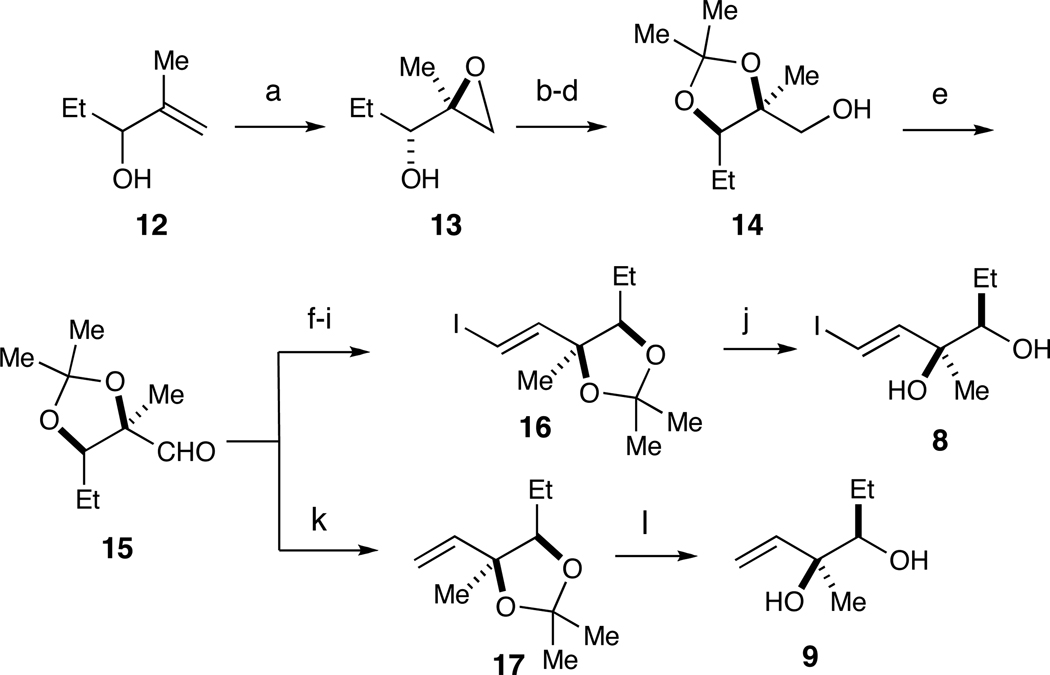
Scheme 2.
Synthesis of fragments 8 and 9 from aldehyde 15.a
a Reagents and conditions: (a) (−)-DIPT, Ti(Oi-Pr)4, t-BuOOH, 32% (92% ee); (b) PivOH, Ti(Oi-Pr)4; (c) Me2C(OMe)2, PPTS; (d) MeLi, 59% over three steps; (e) (COCl)2, DMSO, Et3N; (f) CBr4, Ph3P, CH2Cl2, 65% over two steps; (g) n-BuLi, THF, 90%; (h) cat. AIBN, Bu3SnH, C6H6; (i) I2, CH2Cl2, 80% over two steps; (j) 1N HCl (aq.), 72%; (k) Ph3P=CH2; (l) 1 N HCl (aq.), 53% over three steps from 14.