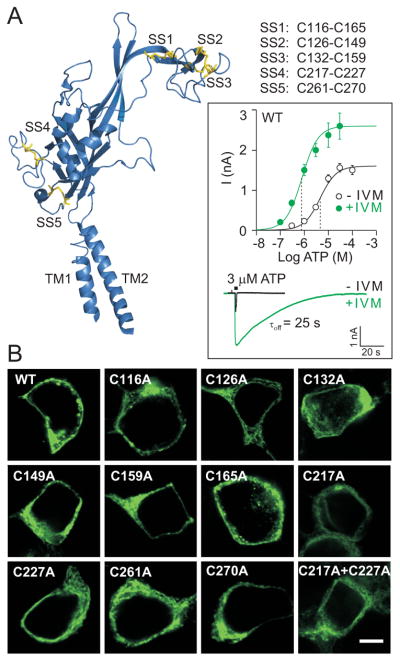
Fig. 1.
Expression and function of wild type (WT) and mutant rat P2X4R in HEK293 cells. (A) Secondary structure organization of one zebrafish P2X4.1 receptor subunit (Kawate et al. 2009) with α-helices, β-sheets and turns in blue and disulfide bonds in yellow (PyMol; pdb 3I5D). Five disulfide bonds are formed between the following conserved cysteine residues (rat P2X4R numbering):116-165 (SS1), 126-149 (SS2), 132-159 (SS3), 217-227 (SS4) and 261-270 (SS5). (Inset) Potentiating effects of IVM on ATP-induced current in HEK293 cells expressing WT-P2X4R. Upper part: concentration dependence of ATP on the peak current amplitude in the absence (open symbols) and presence (closed symbols) of 3 μM IVM. Data points are mean ± SEM values from 5-17 cells per dose. The vertical dotted lines represent the mean EC50 values. Lower part: a sample recording showing the effects of IVM on the peak amplitude and deactivation time (τoff) of current induced by stimulation with 3 μM ATP (2-s pulse). The number represents the τoff value in the presence of IVM. (B) The expression pattern of the WT receptor, ten conserved cysteine-to-alanine single mutants, and the C217A+C227A double mutant of the rat P2X4R in HEK293 cells. Scale bar: 5 μm.