Abstract
Amyotrophic lateral sclerosis is a neurodegenerative disease in which death of motoneurons leads to progressive failure of the neuromuscular system resulting in death frequently within 2–3 years of symptom onset. Focal onset and propagation of the disease symptoms to contiguous motoneuron groups is a striking feature of the human disease progression. Recent work, using mutant superoxide dismutase 1 murine models and in vitro culture systems has indicated that astrocytes are likely to contribute to the propagation of motoneuron injury and disease progression. However, the basis of this astrocyte toxicity and/or failure of motoneuron support has remained uncertain. Using a combination of in vivo and in vitro model systems of superoxide dismutase 1-related amyotrophic lateral sclerosis, linked back to human biosamples, we set out to elucidate how astrocyte properties change in the presence of mutant superoxide dismutase 1 to contribute to motoneuron injury. Gene expression profiling of spinal cord astrocytes from presymptomatic transgenic mice expressing mutant superoxide dismutase 1 revealed two striking changes. First, there was evidence of metabolic dysregulation and, in particular, impairment of the astrocyte lactate efflux transporter, with resultant decrease of spinal cord lactate levels. Second, there was evidence of increased nerve growth factor production and dysregulation of the ratio of pro-nerve growth factor to mature nerve growth factor, favouring p75 receptor expression and activation by neighbouring motoneurons. Functional in vitro studies showed that astrocytes expressing mutant superoxide dismutase 1 are toxic to normal motoneurons. We provide evidence that reduced metabolic support from lactate release and activation of pro-nerve growth factor-p75 receptor signalling are key components of this toxicity. Preservation of motoneuron viability could be achieved by increasing lactate provision to motoneurons, depletion of increased pro-nerve growth factor levels or p75 receptor blockade. These findings are likely to be relevant to human amyotrophic lateral sclerosis, where we have demonstrated increased levels of pro-nerve growth factor in cerebrospinal fluid and increased expression of the p75 receptor by spinal motoneurons. Taken together, these data confirm that altered properties of astrocytes are likely to play a crucial role in the propagation of motoneuron injury in superoxide dismutase 1-related amyotrophic lateral sclerosis and indicate that manipulation of the energy supply to motoneurons as well as inhibition of p75 receptor signalling may represent valuable neuroprotective strategies.
Keywords: amyotrophic lateral sclerosis, astrocytes, microarray, lactate, nerve growth factor
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease in which motoneuron death leads to progressive failure of the neuromuscular system resulting in death within 2–3 years of symptom onset. Approximately 5% of cases with ALS are familial, and 20% of these have been linked to mutations in Cu/Zn superoxide dismutase 1 (SOD1) (Rosen et al., 1993). Transgenic mice carrying mutant forms of SOD1 develop neuromuscular failure similar to human ALS and these murine models have been widely used in ALS research. A compelling body of evidence has accumulated over recent years indicating that glial cells are involved in the pathogenesis of motoneuron injury in ALS, contributing in particular to disease progression after the onset of motoneuron injury (Boillee et al., 2006).
Although targeted expression of mutant SOD1 in astrocytes fails to provoke an ALS phenotype (Gong et al., 2000), the selective silencing of mutant SOD1 expression in astrocytes significantly slowed disease progression in the mouse model (Yamanaka et al., 2008). The use of chimeric mice showed that normal motoneurons develop features of ALS pathology when surrounded by mutant SOD1-expressing glial cells (Clement et al., 2003), strengthening the hypothesis that alteration of the properties of glia by mutant SOD1 contributes significantly to the motoneuron injury occurring in SOD1-related ALS.
Previous reports have also demonstrated that rodent astrocytes expressing mutant SOD1 have toxic effects on cultures of primary motoneurons and embryonic mouse stem cell-derived motoneurons (Nagai et al., 2007). However, differing results have been obtained in vivo when targeted expression of mutant SOD1 is limited to motoneurons (Pramatarova et al., 2001; Lino et al., 2002; Jaarsma et al., 2008), suggesting that mutant SOD1 can trigger motoneuron degeneration, which, in turn, can trigger functional deficits in the surrounding glia.
Thus, it is clear that astrocytes play an important role in ALS, but the way in which the expression of mutant SOD1 alters the properties of astrocytes and the precise role played by astrocytes in motoneuron injury and its propagation in ALS have yet to be elucidated.
The aim of the experimental work described here was to determine the role played by astrocytes in motoneuron injury in SOD1-related ALS. Laser capture microdissection, immunohistochemistry and microarray analysis have been combined to characterize the gene expression profile of astrocytes isolated from the lumbar spinal cord of mice carrying G93A mutant SOD1 (SOD1G93A) and their non-transgenic littermates (NTgG93A) at a presymptomatic stage of disease (60 days). We focused on the presymptomatic time point in order to identify early changes in astrocyte function, prior to the reactive changes occurring in response to motoneuron cell death.
The data show that astrocytes from presymptomatic SOD1G93A mice display significant alterations in lactate and nerve growth factor (NGF) processing. In vitro experiments confirmed the dysregulation affecting lactate and NGF when SOD1G93A astrocytes were co-cultured with non-transgenic motoneurons, but not when monocultured. Our results also indicate that the increased vulnerability of non-transgenic motoneurons grown on SOD1G93A astrocytes is linked to an increase in the ratio between pro-nerve growth factor (pro-NGF) and mature NGF, along with over-expression of the p75 receptor for NGF.
Quantitative polymerase chain reaction analysis showed downregulation of the lactate transporter, solute carrier 16a4 (Slc16a4), in spinal astrocytes from patients with ALS, indicating that lactate release is likely to be impaired in the human disease. Analysis of CSF obtained from patients with ALS confirmed an alteration in the processing of NGF, accompanied by p75 receptor messenger RNA over-expression by spinal motoneurons. This points to an important role for activation of the p75 receptor by pro-NGF and identifies this interaction as a potential therapeutic target.
Taken together, these data confirm the hypothesis that motoneuron degeneration in ALS is a ‘non cell-autonomous’ process. Furthermore, we demonstrate that interaction between mutant SOD1 astrocytes and motoneurons is required for the observed toxic effects and that these findings are relevant to human ALS.
Materials and methods
Murine SOD1 G93A transgenic mouse model
Transgenic mice B6SJL-Tg (SOD1-G93A) 1 Gur/J high copy number backcrossed onto C57Bl/6 J Ola-Hsd (Harlan) for >20 generations were used in this study (Ferraiuolo et al., 2007). Male mice over-expressing the human mutant G93A SOD1 (SOD1G93A), their gender-matched non-transgenic littermates (NTgG93A) and transgenic mice over-expressing human wild-type SOD1 (SOD1WT) and their non-transgenic littermates (NTgWT) were used. Brain and spinal cord were collected at the presymptomatic stage of disease (30-, 40- and 60-day-old mice). All the experiments on mice were performed according to the UK Home Office regulations.
Sectioning and tissue staining for laser capture microdissection
Sixty-day-old mice were perfused with a 30% sucrose solution, and the CNS dissected and frozen. Lumbar spinal cord (10 µm) frozen sections were fixed in acetone at 4°C for 3 min, then incubated in blocking solution for 3 min at room temperature in 10 µl rabbit serum in 5 ml Tris-buffered saline. Sections were incubated with anti-aldehyde dehydrogenase 1L1 (1:50) primary antibody (Abcam) in 1 ml blocking buffer for 3 min at room temperature. Sections were then washed three times in Tris-buffered saline for 10 s and incubated with secondary antibody 1:200 in Tris-buffered saline for 3 min and then washed in Tris-buffered saline three times for 10 s. After incubation in ABC (5 µl buffer A + 5 µl buffer B + 2.5 ml Tris-buffered saline) for 3 min, sections were washed in Tris-buffered saline for 3 min and incubated in diaminobenzidene for 3 min following the manufacturer's protocol (Vector). The reaction was stopped in water for 15 s and sections were dehydrated in a graded alcohol series: 70, 95, 100% for 15 s, left in xylene for 5–10 min and allowed to dry for 30 min.
Microdissection and RNA quality control
From each 60-day-old animal, 1500 astrocytes were isolated using a PixCell II laser capture microdissection system (Arcturus Bioscience). RNA was extracted and the quantity (NanoDrop 1000 Spectrophotometer) and quality (Agilent 2100 bioanalyser, RNA 6000 Pico LabChip) was analysed as previously described (Ferraiuolo et al., 2009).
Amplification and microarray hybridization
Linear amplification of RNA was performed following Eberwine's procedure (Van Gelder et al., 1990) using the Two Rounds Amplification kit (Affymetrix) according to the manufacturer's protocol. Labelled complementary RNA was obtained using the GeneChip Expression 3′-Amplification Reagents for IVT labelling (Affymetrix). Complementary RNA (15 µg) for each of the six GeneChips (three SOD1G93A, three NTgG93A) was fragmented (GeneChip Reagents, Affymetrix) and hybridized onto Mouse Genome 430 2.0 GeneChip (Affymetrix). Microarray chips were washed and scanned and results analysed as previously reported (Ferraiuolo et al., 2009). In order to exclude the presence of contaminating cells types, hybridization data for marker transcripts of oligodendrocytes [e.g. oligodendrocyte myelin glycoprotein (Omg) and oligodendrocyte transcription factor 1 and 2 (Olig1 and Olig2)] and neurons [choline acetyltransferase (ChAT) and netrin (Ntn)] were analysed. Chips were used for the analysis only if these transcripts were ‘absent’ on the pivot table. Transcripts were defined as differentially expressed between the transgenic mice and the littermate controls if there was a 2-fold or greater difference in gene expression level, plus a P < 0.05.
Data analysis
Differentially expressed probes were classified according to Gene Ontology terms (http://www.geneontology.org/). In order to identify specific pathways involved in the development of the disease, the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 (http://david.abcc.ncifcrf.gov/) was used.
Real-time polymerase chain reaction
RNA was extracted from ∼1500 astrocytes and 1000 motoneurons, microdissected as previously described from the spinal cord of six male mice at 60 days, three SOD1G93A and three NTgG93A. RNA (20 ng) was amplified using the two cycle linear amplification protocol (Affymetrix) as described above. Further quantitative and qualitative analysis followed the amplification process and these amplified samples were diluted to the concentration of 12.5 ng/µl for quantitative polymerase chain reaction.
Primer concentrations and other polymerase chain reaction conditions were optimized as previously described (Ferraiuolo et al., 2007). Gapdh was chosen as a housekeeping gene to normalize the quantitative polymerase chain reaction values, because the microarray analysis showed that its expression was stable at every stage of the disease within the transgenic mice and the expressed values obtained from astrocytes of SOD1G93A mice were comparable to those obtained for the corresponding control mice.
Astrocyte cell cultures
Primary cultures of cerebral cortical astrocytes were prepared from SOD1G93A and SOD1WT C57BL/6 newborn mice (1–2 days old). Each brain was treated individually and pups screened for the transgenic SOD1 using qualitative polymerase chain reaction. Astrocytes were grown to confluence in Dulbecco's modified Eagle's medium containing 10% foetal bovine serum in T25 flasks and separated from contaminating microglia through shaking and mild trypsinization (Saura et al., 2003). The isolated astrocytes were then grown to confluence in T75 plates and then plated in 24-well plates at a density of 50 000 cells/well at passage 3 (P3). Three separate preparations per genotype (SOD1G93A, NTgG93A, SOD1WT and NTgWT), each plated in triplicate, were used to establish separated motoneuron co-cultures.
Motoneuron preparations and separated co-cultures
Primary motoneuron cultures were established from E13.5 C57BL/6J mouse embryos as previously described (Henderson, 1995). Motoneuron enrichment was performed by layering the cell suspension onto a 6.4% iodixanol cushion (Axis-Shield) and centrifuging at 500 g for 15 min. The day preceding the motoneuron isolation, 2 mm2 paraffin wax dots were laid onto 1 cm2 glass coverslips. These were then sterilized and coated with 1× poly-d-ornithine solution as previously described (Viviani, 2006). The motoneuron-enriched fraction was plated onto poly-d-ornithine/laminin-coated glass coverslips at 50 000 cells/cm2 in motoneuron medium (neurobasal medium containing B27 supplement, 2% horse serum, 1 ng/ml BDNF, 1 ng/ml GDNF, 5 ng/ml CNTF). Growth factors were purchased from R&D Systems. After 4 h, each coverslip was washed twice in phosphate-buffered saline and laid onto the astrocytes plated in the 24-well plate so that astrocytes and motoneurons would be separated only by neurobasal medium (Supplementary Fig. 3).
Treatments and cell count
Motoneuron co-cultures were set up and motoneurons were counted on Day 1. On Day 2, co-cultures were supplemented with various concentrations of lactate, i.e. 0.5, 1, 2, 5 or 10 mM. Lactate (0.5 mM) was chosen as a starting concentration because adding that quantity of lactate to the media should have equalled the lactate levels present in the physiological media of NTg astrocytes. The concentrations between 1 mM and 10 mM were chosen to test a range of concentrations for beneficial effects as well as toxicity.
Inhibiting antibody p75 (Millipore) was added to culture medium in a single dose at the dilution 1:1000 on Day 3 or 6 after plating motoneurons. Fibroblast growth factor R1 inhibitor PD166866 was added to culture medium at 500 nM concentration (Cassina et al., 2005) on Day 7 after plating motoneurons for 24 h. Glutamate was added to astrocyte culture medium either as an acute exposure (200 µM) for 1 h or as a chronic exposure (2 µM) for 14 days.
Motoneuron counts were performed by taking images of 10 random fields per coverslip at ×20 magnification, corresponding to one-third of the total area of the coverslip. Motoneurons were then counted manually according to the following criteria: a cell body diameter ≥15 µm and the presence of a minimum of three neuritic processes. Three coverslips were counted per preparation and in each experiment at least three different preparations were used per condition.
Lactate measurements
In the media
On the day of the experiment, the coverslips with the motoneurons were removed, leaving the astrocytes only in the 24-well plate. The culture medium (complete neurobasal) was replaced with 500 µl serum-free, phenol red-free Dulbecco's modified Eagle's medium containing 4.5 g/l glucose, supplemented with 0.06 g/l of penicillin, 0.1 g/l of streptomycin and 1× l-glutamine. The astrocytes were incubated for 4 h at 37°C in a water-saturated atmosphere containing 5% CO2/95% air. After 4 h, the supernatant was collected and centrifuged at 400g for 4 min at 4°C and stored on ice. Astrocyte-conditioned medium (50 µl) was used for measurement of lactate according to the manufacturers’ instructions (Lactate Colorimetric kit, Abcam). Lactate levels were normalized to cell numbers.
In the spinal cord
Six SOD1G93A mice and six non-transgenic littermates at 30, 40 and 60 days of age were culled by cervical dislocation, spinal cords were divided into lumbar and cervical/thoracic regions, each region was longitudinally divided into two and snap frozen in liquid nitrogen. On the day of the experiment, half lumbar spinal cord was homogenized into 300 µl of lactate assay buffer (Lactate Colorimetric kit, Abcam) and centrifuged at 4°C at 10 000g for 4 min. Samples were tested according to the manufacturer's protocol removing NADH/NADPH background.
Nerve growth factor measurements
NGF levels were measured in astrocyte growth medium (Dulbecco's modified Eagle's medium) on the day before adding the motoneurons to the separated co-culture (Day 6 after plating) and 48 h after removing the motoneurons from the co-culture (Day 23 after plating). To avoid cross-reaction with the high levels of neurotrophic factors present in the neurobasal medium, on the 14th day of separated co-culture, motoneurons were removed from the well and complete neurobasal was replaced with 500 µl of fresh Dulbecco's modified Eagle's medium and 10% foetal bovine serum for 48 h. This time point was chosen after a time course ELISA assay showing that NGF reaches detectable levels in the media 48 h after media change (data not shown). The media was removed and centrifuged at 400 g for 4 min at 4°C to remove cellular contamination.
Human CSF was collected on ice and immediately centrifuged at 400 g for 4 min at 4°C to remove cells prior to storage in liquid nitrogen. On the day of the experiment, CSF was thawed in ice. NGF content in the media and in human CSF was analysed using the NGF Emax ImmunoAssay System (Promega) following the manufacturer's instructions.
Nerve growth factor immunoprecipitation
Aliquots of 1 ml of astrocyte-conditioned neurobasal medium were collected on ice and centrifuged at 400g for 4 min to remove cellular debris. Supernatants were incubated with 5 μg each of monoclonal anti-NGF antibody (Promega) or 5 μg of control antibody (rabbit anti-rat IgG) in the presence of 20 μl 50% protein G agarose bead slurry for 4 h at 4°C with gentle mixing. The beads were collected by centrifugation at 1000g at 4°C, and the supernatant was saved for immunoblotting or filter sterilized for motoneuron media. After removal of the supernatant, the resin was washed three times with wash buffer (20 mM Tris–HCl, pH 8, 200 mM NaCl, 0.2 mM EDTA, 10% glycerol and 0.1% IGEPAL) and boiled in 1× sodium dodecyl sulphate gel loading buffer. Control and NGF-depleted extracts, as well as the immunoprecipitated material, were analysed by both western blotting and ELISA.
CSF was thawed on ice and the immunoprecipitation protocol was performed as described above. Pellets were resuspended in 20 µl of loading buffer and both immunodepleted CSF and pellets were used for both ELISA and western blotting.
Fibroblast growth factor 1 measurements
Fibroblast growth factor 1 levels were measured in astrocyte growth medium (Dulbecco's modified Eagle's medium) on the day before adding the motoneurons to the co-culture (Day 6 after plating) and in motoneuron growth media (neurobasal) 7 days after motoneuron plating. The medium was removed and centrifuged at 400g for 4 min at 4°C to remove cellular contamination. Fibroblast growth factor 1 content was analysed using the fibroblast growth factor acidic Quantikine ELISA Kit (R&D) following the manufacturer's instructions.
Western blotting
After NGF immunoprecipitation, pellets were diluted 1:50 in loading buffer and supernatants were filtered using 50 kDa columns to remove abundant heavy proteins from the serum and concentrated by centrifugation. Both pellet and supernatants were run on a 12% acrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane. The portion of the membrane containing proteins above 23 kDa was tested for pro-NGF using the rabbit monoclonal anti-pro-NGF (Millipore) 1:1000 and the horseradish peroxidase-conjugated anti-rabbit antibody (Dako) 1:2500 recognizing pro-NGF (33 kDa). The portion of the membrane containing proteins <23 kDa was blotted with the rat anti-NGF monoclonal antibody (Promega) 1:1000 and the horseradish peroxidase-conjugated anti-rat antibody (Promega) 1:2500. CSF from patients was thawed on ice and immunoprecipitated and pellets blotted as described above.
P75 receptor quantification
Three 14-day-old motoneuron and astrocyte co-cultures per experimental condition (i.e. basal conditions, 1 mM lactate addition and NGF depletion) per genotype (i.e. SOD1G93A and NTg astrocytes) were fixed in 4% paraformaldehyde and immunolabelled using the mouse monoclonal p75 antibody (Abcam) 1:1000 and the fluorescent secondary antibody goat anti-mouse Alexa 555 (Invitrogen) 1:200. Antibodies were titrated and the concentration used was chosen in the range of subsaturation. When collecting images, all parameters were kept constant and set in order not to reach signal saturation (exposure 30 ms, gain 59% and offset 0%).
For each coverslip, images of 20 motoneurons were taken using the Openlab software (Improvision) and the fluorescence intensity per cell was analysed using the particle analysis function of ImageJ programme developed by Wayne Rasband (NIH, http://rsb.info.nih.gov/ij/). Further calculations and statistical analyses were performed using Excel (Microsoft Corporation) and Prism software (GraphPad Software Inc.) taking into account 60 cells per condition per genotype arising from three different coverslips.
Human spinal cord material
Cervical spinal cord blocks from three SOD1-related cases with ALS (one p.E100G and two p.I113T) and three neurologically normal control cases were obtained from the Sheffield Brain Tissue Bank. Spinal cord sections were prepared, and ∼500 motoneurons were isolated as described previously (Kirby et al., 2011). Eight hundred astrocytes per spinal cord were also isolated from the same tissues using the methods described for the murine material. RNA was extracted and converted to complementary DNA and used for quantitative polymerase chain reaction as described above.
Statistical tests
Microarray results were analysed using the programme Array Assist 4.0 (Iobion). The statistical test applied to identify differentially expressed genes after normalization with the algorithm PLIER 16 was the unpaired two-tailed Student t-test. Probes presenting a coefficient of variation ≥50% within the same genetic group were excluded from the analysis.
For all the other experiments, the statistical analysis was performed using the Prism software (GraphPad Software Inc.). The statistical tests used were the unpaired two-tailed t-test for two group comparisons and one-way ANOVA for multiple group comparisons followed by Tukey's multiple comparison test.
Results
Mutant SOD1-induced changes in the astrocyte transcriptome
Astrocytes stained using the antibody anti-aldehyde dehydrogenase 1L1 (Cahoy et al., 2008) (Supplementary Fig. 1) were isolated using laser capture microdissection from the lumbar spinal cords of three SOD1G93A mice and three NTgG93A at 60 days and their transcription profiles were generated and compared using Array Assist 4.0.
Transcripts (n = 583) showed increased expression and 526 showed decreased expression in the mutant SOD1 astrocytes as determined by a fold change ≥2 and a P ≤ 0.05. Gene ontology classification revealed that these transcripts mainly belong to energy metabolism, signalling, cell cycle, immune response and exo/endocytosis (Supplementary Table 1). Particular attention was directed to expression changes in genes involved in metabolism and signalling, as they showed a clear correlation with previously reported results obtained from motoneurons isolated from SOD1G93A mice at the same stage of disease (60 days) (Ferraiuolo et al., 2007).
Metabolic dysfunction and involvement of the lactate shuttle
Comparison between astrocytes from the spinal cord of SOD1G93A mice and NTgG93A showed differential expression of multiple transcripts involved in carbohydrate metabolism. Of particular interest is the downregulation of the rate-limiting enzyme phosphoglycerate kinase 1 (Pgk1; −2.2) involved in glycolysis, isocitrate dehydrogenase 3 (NAD+) alpha (Idh3a; −2.0) involved in tricarboxylic acid metabolism and ATP synthase mitochondrial F0 complex F1 (Atp5f1; −3.5). Also of interest is the downregulation of the solute carriers 19a3 (Slc19a3; −2.3) and 16a4 (Slc16a4; −2.55), which are involved in thiamine uptake and lactate release from the cell, respectively. Slc16a4 is prominently expressed in astrocytes (Pierre and Pellerin, 2005). Lactate release is linked to the activity of the glutamate transporters, which co-transport glutamate and Na+. The microarray analysis revealed downregulation of the glutamate-aspartate transporter Glast (also known as Slc1a3; −3.2) and of the Na+/K+ ATPase alpha-1 polypeptide (Atp1a1; −2.7), both indicating that the lactate shuttle might be impaired. Slc16a4 and Atp1a1 expression changes in laser-captured astrocytes have been confirmed by quantitative polymerase chain reaction (Supplementary Fig. 2A and B).
Altered expression of neurotrophic factors
Transcription profile analysis highlighted the upregulation of growth factors and receptors such as fibroblast growth factor receptor 4 (Fgfr4; +2.0) and transforming growth factor alpha (Tgfa; +4.0). Interestingly, nerve growth factor b (Ngfb) is also upregulated (+2.7), as confirmed by quantitative polymerase chain reaction (Supplementary Fig. 2C).
The toxic function of Ngfb on motoneurons has been related to its binding with the receptor p75 (Pehar et al., 2007) and interaction of the latter with its associated protein, Ngfap1 (Yi et al., 2003). The expression of both p75 and Ngfrap1 was upregulated (+2.3 and +2, respectively) in motoneurons isolated from the spinal cord of the same three SOD1G93A mice and NTgG93A used for the astrocyte analysis (Supplementary Fig. 3A and B).
SOD1G93A mice display lower levels of spinal cord lactate from the age of 40 days
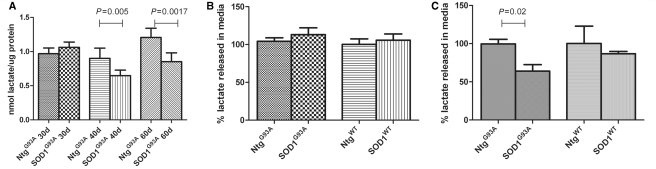
The decreased expression of the astrocytic lactate transporter Slc16a4 in the presence of mutant SOD1 is predicted to cause impairment of lactate release. To investigate this, we measured lactate levels in the spinal cord of six SOD1G93A mice and six NTgG93A at 60 days and a significant 30% reduction in lactate content was detected (P = 0.0017) (Fig. 1A). At 30 days, there was no detectable difference between SOD1G93A mice and NTgG93A, but at 40 days a significant 20% decrease in spinal cord lactate is detectable (P = 0.005) (Fig. 1A).
Figure 1.
Lactate levels were measured in spinal cord homogenates from SOD1G93A mice and NtgG93A at 30, 40 and 60 days of age (A) (n = 6 and bars = SD, two-tailed t-test) and in the culture media after growing astrocytes from SOD1G93A and SOD1WT and respective non-transgenic littermates (NtgG93A and NtgWT) in monoculture for 21 days (B) and in ‘separated co-cultures’ with non-transgenic motoneurons for 14 days (C) (n = 3, each n is a triplicate, and bars = SD). Results are expressed as percentage of the lactate released in the media by non-transgenic astrocytes.
In order to investigate whether the motoneuron lactate influx transporter expression was involved in the metabolic stress observed in motoneurons isolated from SOD1G93A mice and NTgG93A at 60 days (Ferraiuolo et al., 2007), quantitative polymerase chain reaction analysis was performed on messenger RNA isolated from the motoneurons of the same SOD1G93A mice and NTgG93A used for the astrocyte analysis. The results showed that the monocarboxylate transporter 1 (Mct1 also known as Slc16a1) was upregulated (+2.8; P = 0.0001) in transgenic mice (Supplementary Fig. 3C), while Slc16a4 was not altered (data not shown).
Impairment in the astrocyte–motoneuron lactate shuttle is triggered by motoneurons and affects the spinal cord of patients with amyotrophic lateral sclerosis
Spinal cord measurements are taken from a heterogeneous population of cells. In order to determine the contribution of astrocytes in this complex system, primary astrocyte monocultures from SOD1G93A, SOD1wt mice and their respective non-transgenic littermates were established and either tested for lactate release in the media after 21 days in monoculture or following growth as a monoculture for 7 days, followed by interaction with non-transgenic motoneurons for 14 days as a separated co-culture (Supplementary Fig. 4). Interestingly, the same levels of lactate were found in the medium of monocultured SOD1G93A, NTg and SOD1WT astrocytes (Fig. 1B). However, SOD1G93A astrocytes secrete 35% less lactate compared with NTg and SOD1WT astrocytes when grown in separated co-cultures with motoneurons (Fig. 1C). Consistent with this finding, after 14 days in co-culture, SOD1G93A astrocytes show transcriptional downregulation of Slc16a4 (Supplementary Fig. 5A), while motoneurons demonstrate upregulation of Slc16a1 (Supplementary Fig. 5B), confirming the in vivo data.
Remarkably, the lactate transporter Slc16a4 was found to be significantly downregulated (P = 0.0016) also in spinal astrocytes isolated from three patients with ALS carrying SOD1 mutations compared with control cases (Supplementary Fig. 5C).
SOD1G93A astrocytes do not support motoneuron viability as well as NTg and lactate can rescue this deficiency
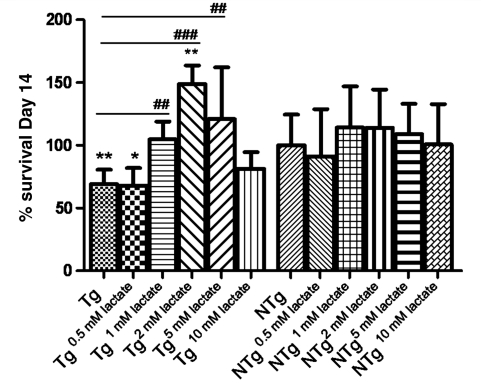
In order to determine whether lower levels of lactate may be of pathophysiological significance in the motoneuron injury occurring in ALS, we tested the survival of primary non-transgenic motoneuron co-cultured on a monolayer of SOD1G93A and NTg astrocytes with and without the addition of lactate to the media. Primary astrocytes were plated and grown for 7 days as a monoculture, and on the seventh day non-transgenic motoneurons were plated on the astrocytic monolayer. The number of motoneurons per well was assessed on Day 1 and 14 post-plating, and co-cultures were maintained in complete neurobasal medium either without lactate supplements or with lactate to a final concentration of 0.5, 1, 2, 5 or 10 mM from Day 2 post-plating. On Day 1, there was no significant difference between the number of motoneurons surviving on the different astrocytic monolayers. In contrast, at Day 14, there was a 38% reduction (P = 0.0015) in motoneuron viability in SOD1G93A astrocytes co-cultures, showing that mutant SOD1 astrocytes are less effective in supporting motoneuron survival in vitro (Fig. 2). Lactate supplements did not affect the co-cultures with NTg astrocytes, while in co-cultures with SOD1G93A astrocytes, motoneurons were rescued when 1, 2 and 5 mM lactate was added (1 mM P = 0.0003, 2 mM P < 0.0001, 5 mM P = 0.0024) (Fig. 2). Lactate concentrations of 0.5 and 10 mM did not improve motoneuron survival.
Figure 2.
Motoneuron survival after 14 days of co-culture with SOD1G93A (Tg) or NTg astrocytes (n = 3, each n is a triplicate, bars = SD, one-way ANOVA plus Tukey's multiple comparison test). Results were normalized to motoneuron survival grown on NTg astrocytes without lactate treatment. Statistical analysis was performed comparing all groups to motoneuron survival on SOD1G93A (significance = #; where ## = <0.01; ###= <0.001) or NTg (significance =*; where *= <0.05; **= <0.01) astrocytes under basal conditions. SOD1G93A astrocytes increase motoneuron death by 38% (**P < 0.01) compared with NTg astrocytes under basal conditions. The addition of 0.5 mM lactate has no effect, while 1 mM lactate completely rescues motoneuron viability on SOD1G93A astrocytes. Lactate (2 mM) concentration improves motoneuron survival on SOD1G93A astrocytes compared with motoneuron survival on NTg astrocytes untreated (**P < 0.01). NTg = non-transgenic; Tg = transgenic.
SOD1G93A astrocytes release increased levels of pro-nerve growth factor into the media compared with non-transgenic astrocytes
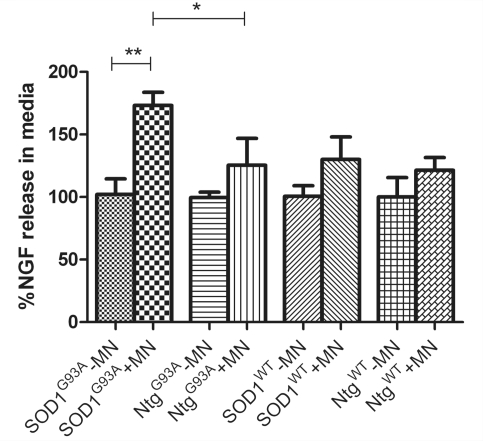
In order to further investigate the prediction based on our microarray results that astrocytes expressing SOD1G93A produce and release more Ngf than NTg and SOD1WT astrocytes, the levels of total Ngf (pro-Ngf plus mature Ngf) in the culture media of both monocultured astrocytes and ‘separated co-cultures’ with non-transgenic motoneurons were measured. The results showed that the level of Ngf secreted by astrocyte monocultures does not vary with time (data not shown). In contrast, Ngf measurements in the media before and after plating motoneurons onto the astrocyte showed that, in co-culture, non-transgenic motoneurons stimulated a 20–30% increase in Ngf secretion by NTgG93A, SOD1WT and NTgWT astrocytes (Fig. 3). Interestingly, SOD1G93A astrocytes release 70% more Ngf after co-culture with non-transgenic motoneurons, corresponding to ∼40% more secreted Ngf compared with NTgG93A, SOD1WT and NTgWT under identical conditions (Fig. 3).
Figure 3.
Ngf release in the media normalized to the levels found in astrocyte monocultures. SOD1G93A astrocytes release 70% more Ngf after being co-cultured with motoneurons (**P = 0.0016), and they release 38% more than NtgG93A (*P = 0.025), 33% more than SOD1WT (P = 0.022) and 43% more than NtgWT (P = 0.0035) in the same conditions (n = 3, each n is a triplicate, bars = SD, two-tailed t-test). MN = motoneuron.
This increase in Ngf secretion by mutant SOD1 astrocytes is likely to affect co-cultured motoneurons as demonstrated by a significant upregulation of p75 and Ngfrap1 expression by motoneurons after 14 days in co-culture with SOD1G93A astrocytes (Supplementary Fig. 6).
Total nerve growth factor depletion improves survival in motoneurons co-cultured on SOD1G93A astrocytes, but is harmful to motoneurons co-cultured on non-transgenic astrocytes
In order to investigate the effect of total Ngf on motoneurons grown in co-culture with SOD1G93A or NTg astrocytes, co-cultures were set up as described before, motoneurons were counted on Day 1 and grown for 14 days. Every 48 h culture media was replaced by Ngf immunodepleted media collected from a ‘twin’ co-culture set up in parallel to the one analysed. In this way, one co-culture was used as source of media and the other was used to evaluate the effects of Ngf depletion on co-cultured motoneurons. This approach was used to avoid further media replacements while going through the immunodepletion protocol.
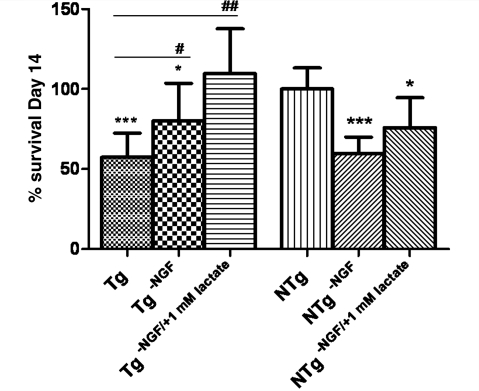
After 14 days, motoneuron counting revealed that Ngf depletion is beneficial for motoneuron grown on SOD1G93A astrocytes, leading to a significant 20% increase in survival (P = 0.048) compared with basal conditions, while it is harmful for motoneuron grown on non-transgenic astrocytes, leading to a dramatic 40% decrease in survival (P = 0.0002) (Fig. 4).
Figure 4.
Motoneuron survival after 14 days of co-culture with SOD1G93A (Tg) or NTg astrocytes under basal conditions, with Ngf depletion and with Ngf depletion combined with 1 mM lactate supplementation (n = 3, each n is a triplicate, bars = SD, one-way ANOVA). Results were normalized to motoneuron survival grown on NTg astrocytes under basal conditions. Statistical analysis was performed comparing all groups to motoneuron survival on SOD1G93A (significance = #, where #= < 0.05; ##= < 0.01) or NTg (significance =*, where *= < 0.05; ***= < 0.001) astrocytes under basal conditions. SOD1G93A astrocytes increase motoneuron death by 40% (P = 0.0004, two-tailed t-test) compared to NTg astrocytes under basal conditions. Ngf depletion partially rescues motoneuron viability on SOD1G93A astrocytes (20% increase, P<0.05), and, when combined with 1 mM lactate in the media, motoneuron survival is totally rescued. Motoneuron survival is significantly reduced by Ngf depletion in co-cultures with NTg astrocytes (40% increased death, P<0.001); lactate partially rescues motoneuron loss reducing motoneuron death by 15% (P < 0.05). NTg = non-transgenic; Tg = transgenic.
Ngf depletion was also combined with 1 mM lactate supplementation, which resulted in complete rescue of motoneuron survival in the co-cultures with SOD1G93A astrocytes, whereas it improved, but was unable to completely counteract, the toxic effects of Ngf depletion in co-cultures with NTg astrocytes (Fig. 4).
Pro-nerve growth factor accounts for the increase of total nerve growth factor detected in motoneuron co-cultures with SOD1G93A astrocytes
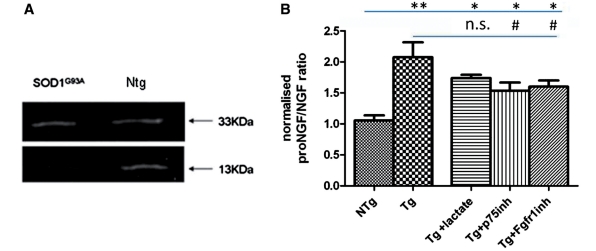
The contrasting effects obtained when depleting total Ngf from co-cultures with NTg or SOD1G93A astrocytes prompted us to determine whether this divergent outcome was related to the presence of different species of Ngf in the two co-cultures. Western blot analysis revealed that SOD1G93A astrocytes secrete higher levels of pro-NGF, resulting in a pro-NGF (33 kDa)/mNgf (13 kDa) ratio 2-fold greater (P = 0.01) than that obtained from co-culture media of normal astrocytes (Fig. 5).
Figure 5.
Western blot (A) and densitometry (B) of total Ngf immunoprecipitated from the media collected from SOD1G93A and Ntg astrocyte co-cultured with non-transgenic motoneurons for 14 days. Western blotting showed the presence of two forms of Ngf: pro-NGF (33 kDa), and mutant Ngf (13 kDa). Densitometry revealed that in the medium from co-cultures of SOD1G93A astrocytes with non-transgenic motoneurons the pro-NGF/mutant Ngf ratio is 2-fold higher (P = 0.001, two-tailed t-test) than in co-cultures with non-transgenic astrocytes (n = 6; error bar = SD). (B) Densitometry results for pro-NGF/NGF ratio when co-cultures are treated with lactate, p75 inhibitor and fgfr1 inhibitor (n = 3; error bar = SD, one-way ANOVA). Statistical analysis was performed comparing all groups to Ntg (significance =*, where *= < 0.05; **=<0.01) or SOD1G93A (significance = #, where #= <0.05. n.s. = not significant) Levels of pro-NGF/NGF were also analysed for Ntg co-cultures, but there was no significant difference between treatments (data not shown).
p75 Nerve growth factor receptor expression and localization is associated with motoneuron vulnerability to cell death
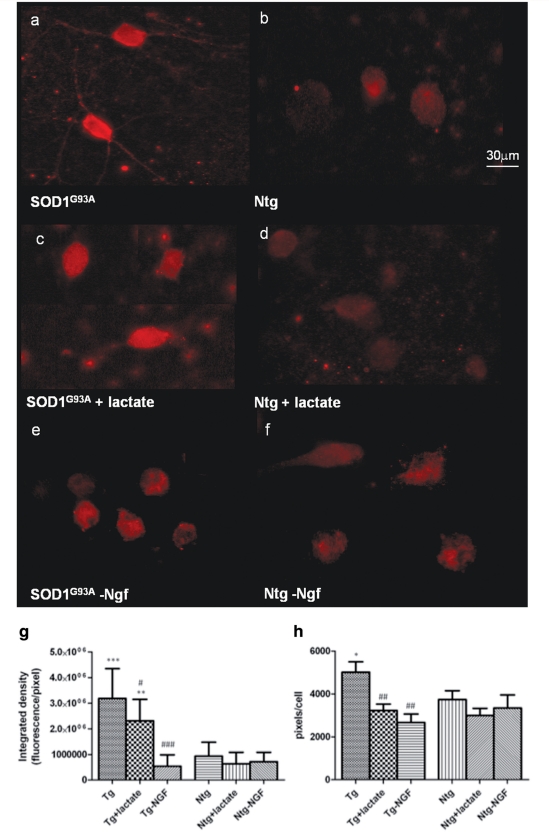
In order to determine whether higher levels of pro-NGF had altered the expression pattern of its receptor on non-transgenic motoneurons, p75 expression was investigated in astrocyte–motoneuron co-cultures. Our results showed that motoneurons grown on SOD1G93A astrocytes express 3.5 times more p75 than motoneurons grown on NTg astrocytes (P < 0.0001), and the cellular area labelled for the p75 receptor is 1.3 times higher (Fig. 6a, b and g–h). Motoneurons grown on SOD1G93A astrocytes, in fact, express p75 not only on the cell body, but also on axons and dendrites (Fig. 6a).
Figure 6.
p75 expression in motoneuron cultured for 14 days on SOD1G93A or Ntg astrocytes under basal conditions (a and b), with 1mM lactate supplementation (c and d) and Ngf depletion (e and f). p75 fluorescence has been quantified and expressed as integrated density (g) in order to capture the quantity of p75 expressed and pixel/cell (h) in order to quantify the area positive for p75. Statistical analysis was performed comparing all groups to p75 expression in motoneurons grown on Ntg (significance =*, where *=<0.05; **= < 0.01; ***= < 0.001) or SOD1G93A astrocytes (significance = # where #= <0.05; ##= < 0.01; ###= < 0.001) under basal conditions (n = 60, bars = SD, one-way ANOVA). Our results show that motoneurons cultured under the influence of SOD1G93A astrocytes show expression of p75 within dendrites and axons as well as the perikarya (a), which is absent in all other conditions (b–f). Consistently, fluorescence quantification (g–h) shows that p75 intensity is significantly higher in normal motoneurons co-cultured for 14 days with SOD1G93A astrocytes compared to motoneurons co-cultured with Ntg astrocytes (P < 0.0001) and the area positive for p75 is significantly higher (P < 0.05). Both lactate supplementation and NGF depletion cause a significant reduction in the area positive for p75, bringing it to levels comparable to those observed in non-transgenic co-cultures. The intensity of p75, nevertheless, reaches normal levels in motoneurons cultured with transgenic astrocytes only after NGF depletion (P < 0.0001).
Interestingly, p75 receptor expression undergoes a significant 30% reduction (P = 0.02) when motoneurons are co-cultured on SOD1G93A astrocytes in the presence of 1 mM lactate in the medium (Fig. 6g and h), and reaches normal levels when the culture media has been depleted of Ngf (Fig. 6e–h).
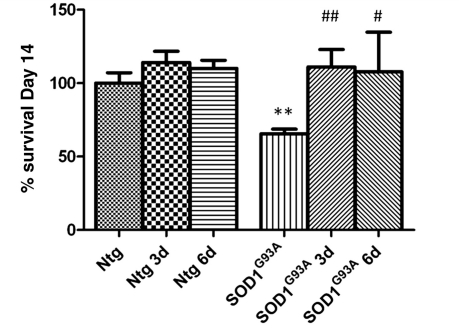
Subsequently, we investigated whether a p75 receptor blocking antibody could rescue normal motoneurons co-cultured with SOD1G93A astrocytes. SOD1G93A and NTg astrocyte co-cultures were established as described above. A p75 inhibiting antibody was added to culture media in a single dose 3 or 6 days after motoneuron plating. After 14 days, our results show that in both conditions p75 receptor blockade completely rescues motoneuron viability (Fig. 7), and leads to a significant decrease in the ratio pro-NGF/NGF in the medium, while lactate treatment does not alter the fraction of the two forms of NGF (Fig. 5B).
Figure 7.
Motoneuron survival after 14 days of co-culture with Ntg or SOD1G93A astrocytes under basal conditions and with p75 inhibitory antibody addition to the media at 3 or 6 days after co-culture (n = 3, each n is a triplicate, bars = SD, one-way ANOVA). Results were normalized to motoneuron survival grown on Ntg astrocytes under basal conditions. Statistical analysis was performed comparing all groups to motoneuron survival on Ntg (significance =*) or SOD1G93A (significance = #) astrocytes under basal conditions. SOD1G93A astrocytes increase motoneuron death by 40% (**P = 0.0015, two-tailed t-test) compared to Ntg astrocytes under basal conditions. Motoneuron survival is totally rescued when the p75 inhibitory antibody is added at either 3 (##P < 0.01) or 6 days (#P < 0.05) after co-culture.
Relevance to human amyotrophic lateral sclerosis
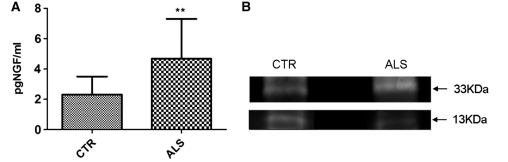
ELISA analysis of CSF from 13 patients with sporadic ALS and 13 non-neurological controls revealed that patients with ALS display significantly higher levels of total NGF (Fig. 8A). In those samples with levels of total NGF within the range of sensitivity for western blot analysis, the ratio between pro-NGF and mature NGF is on average 40% higher in the CSF of patients with ALS compared with controls (Fig. 8B).
Figure 8.
NGF quantification in CSF from non-neurological controls and patients with ALS using ELISA (A) for total NGF (pro-NGF + mutant NGF) and western blotting (B). The ELISA test (A) revealed that patients with ALS have CSF levels of NGF significantly higher than controls (P = 0.009; n = 13, error bar = SD, two-tailed t-test). Western blot analysis (B) of NGF immunoprecipitated from CSF showed that in samples where NGF levels were within the range of detection for western blotting (4–8 pg/ml), pro-NGF (33 kDa) is typically more abundant in CSF from patients with ALS than non-neurological controls (CTR). In contrast, NGF (13 kDa) is less abundant. This results in a ratio pro-NGF/NGF ∼40% higher in patients with ALS compared with controls.
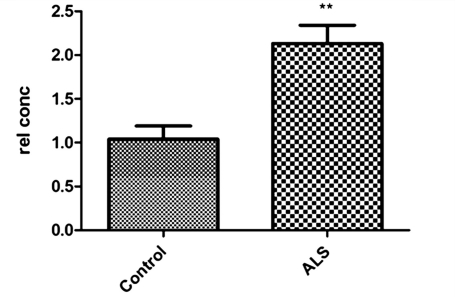
In order to determine the involvement of p75 in the motoneuron degeneration process, the messenger RNA expression level of p75 was investigated in motoneurons isolated from the spinal cord of three patients with ALS carrying SOD1 mutations and three controls. Quantitative polymerase chain reaction revealed that motoneurons from patients with ALS express significantly more p75 receptor messenger RNA (+2.5; P = 0.001) (Fig. 9).
Figure 9.
Quantitative polymerase chain reaction to quantify the expression of p75 in motoneurons isolated from the spinal cord of control individuals and patients with ALS (n = 3 and bars = SD, two-tailed t-test). Results are expressed as relative concentrations (rel con) after normalization to Gapdh levels. p75 is significantly upregulated in motoneurons from patients with ALS (**P = 0.0018).
Glutamate regulates lactate and nerve growth factor release
Acute (200 µM for 1 h) and chronic (2 µM for 14 days) treatment with glutamate leads to significant increased levels of both lactate and total NGF in both transgenic and non-transgenic astrocyte monocultures in the culture media (Supplementary Fig. 7), but there is no significant change in the ratio between pro-NGF and NGF or decrease in lactate release from transgenic astrocytes (data not shown). Interestingly, the levels of lactate released in the media are significantly lower than the levels achieved when motoneurons are in the culture. In contrast, NGF levels are lower compared with astrocyte–motoneuron co-cultures (Supplementary Fig. 7).
Fibroblast growth factor receptor 1 inhibition leads to decrease in pro-nerve growth factor/nerve growth factor ratio
An increase in Fgf1 and Fgf1 receptor have been associated with ALS pathology and with increased NGF release (Cassina et al., 2005). In order to investigate the hypothesis that Fgf1 could be the factor leading to pro-NGF/NGF and lactate shuttle dysregulation, we measured Fgf1 levels in astrocyte monocultures and astrocyte–motoneuron co-cultures. Fgf1 levels significantly increase when motoneurons are added to the in vitro system (from 2 pg/ml in astrocyte monocultures to 5 pg/ml in motoneuron co-cultures), but there was no difference between transgenic and non-transgenic co-cultures (data not shown). Nevertheless, 7 days after plating motoneurons, the fibroblast growth factor R1 inhibitor PD166866 was added to the co-culture for 24 h and pro-NGF levels measured 72 h later. At this time point, motoneuron death was almost total in both transgenic and non-transgenic co-cultures, but pro-NGF levels were 25% lower than in the transgenic untreated co-cultures (Fig. 5), and PD166866 was unable to rescue the impairment in lactate release.
Discussion
In recent years, the use of mixed co-cultures and chimeric mice has highlighted the importance of non-neuronal cells in the motoneuron injury occurring in ALS (Gong et al., 2000; Pramatarova et al., 2001; Lino et al., 2002; Boillee et al., 2006; Nagai et al., 2007; Yamanaka et al., 2008). One of the difficulties in determining the contribution of astrocytes and microglia to disease onset and progression is the complexity of interactions occurring between non-neuronal cells and motoneurons in vivo. On the other hand, using an in vitro model system, where functional effects can be investigated, may represent too simplified an experimental system. In order to begin to understand the complex cross-talk between astrocytes and motoneurons, we analysed the transcriptome of astrocytes isolated from the spinal cord of presymptomatic SOD1G93A compared with control mice. Emerging single pathways of relevance were then investigated using in vitro models. We were able to compare the altered gene expression in astrocytes with data obtained from motoneurons at the same disease stage, as previously reported (Ferraiuolo et al., 2007). This enabled us to focus attention on dysregulated pathways involved in the interaction between astrocytes and motoneurons. The use of ‘separated co-cultures’ was a helpful new tool allowing the investigation of functional changes triggered by the interaction between mutant SOD1 astrocytes and normal motoneurons. Finally, using our bank of human biosamples, we were able to investigate the relevance of the findings in the cellular and murine experimental model systems in relation to human ALS.
Lactate is an important source of energy for motoneurons and, according to the lactate shuttle hypothesis, astrocytes are the main providers of this substrate (Pellerin and Magistretti, 1994). In this model, motoneurons stimulate aerobic glycolysis in astrocytes through glutamate release. Sodium-coupled re-uptake of glutamate by astrocytes results in the activation of the Na+/K+-ATPase pump that consumes the ATP produced by phosphoglycerate kinase (Pgk). This triggers glucose uptake and its glycolytic processing, resulting in the release of lactate from astrocytes. Lactate can then contribute to the activity-dependent fuelling of the neuronal energy demands associated with synaptic transmission (Pellerin et al., 2007).
The microarray data in this report suggest that astrocytes isolated from presymptomatic SOD1G93A mice undergo a general downregulation of all the crucial transcripts involved in the lactate shuttle pathway, including the glutamate transporter Glast, Na+/K+-ATPase, Pgk and the lactate efflux transporter Slc16a4. Upregulation by motoneurons of transcripts involved in the tricarboxylic acid cycle and respiratory chain, as well as lipid metabolism, suggests a condition of energy deprivation that is likely to represent a reaction to the lack of substrate provision from astrocytes (Ferraiuolo et al., 2007). Lactate measurements in the spinal cord of transgenic mice confirmed that SOD1G93A mice develop this metabolic impairment between the age of 30 days, when lactate levels are still normal, and 40 days, when there is a 20% decrease in lactate, with a further decrease by 30% at 60 days. Recent reports indicate that initial evidence of denervation in mutant SOD1 mice can be detected at ∼40 days (Hegedus et al., 2007; Hayworth and Gonzalez-Lima, 2009), providing an interesting link between the onset of denervation and motoneuron metabolic dysfunction.
In agreement with the lactate shuttle model, our in vitro data show that astrocytes expressing SOD1G93A, when cultured as a monoculture, do not show any alteration in lactate secretion, while the addition of non-transgenic motoneurons to the cultures, probably through glutamate release, triggers the impairment. These data suggest that the metabolic impairment observed does not require the expression of SOD1G93A in the motoneurons; however, these cells are responsible for triggering the dysfunction. The upregulation of the lactate and the glucose transporters (Slc16a1 and Glut3) that was observed in vivo in motoneurons from SOD1G93A mice was also reproduced in vitro, when non-transgenic motoneurons were grown on SOD1G93A primary astrocytes. This indicates that dysregulated energy provision by mutant SOD1 astrocytes occurs in response to motoneurons, regardless of the genotype. Interestingly, a previous report has also shown that normal motoneurons grown on transgenic astrocytes develop mitochondrial membrane potential abnormalities, which could also be related to lack of energy support (Bilsland et al., 2008).
The finding that spinal astrocytes from patients with ALS also show downregulation of Slc16a4 supports the relevance to the human disease of the impairment affecting lactate metabolism and the trafficking of lactate between motoneurons and astrocytes.
Astrocytes from SOD1G93A mice also show high expression of trophic factors, including Ngf. NGF is secreted by astrocytes as pro-NGF (33 kDa) and this is then cleaved in the extracellular space to produce mature NGF (13 kDa), through a complex mechanism involving several proteases (Bruno and Cuello, 2006; Lim et al., 2007). It was previously reported that mature NGF represents only a minor fraction of total extracellular NGF (Bierl et al., 2005). Mature NGF is known to bind the TrkA receptor with high affinity, leading to neuronal differentiation and survival (Moubarak et al., 2010), and binds to the p75 receptor with low affinity. Pro-NGF, however, preferentially activates the p75 receptor. During development, the p75 receptor plays a key role in axonal growth and remodelling (Boyd and Gordon, 2001), but in adult healthy motoneurons its expression becomes undetectable. It is known that p75 expression by mature neurons can be upregulated in pathological conditions (Dechant and Barde, 2002), and can lead to apoptosis through activation of NfkB, p53 and Bax (Mukai et al., 2003). It is clear that the activation of a survival, or apoptotic signalling cascade, depends on a delicate balance between the ratio of pro-NGF and mature NGF in the extracellular space, the rate at which pro-NGF is processed into mature NGF, as well as the relative expression levels of the TrkA and p75 receptors. It has been previously reported that transgenic mice expressing mutant SOD1 produce in the spinal cord more total NGF than non-transgenic mice, and that NGF toxicity can be prevented by inhibiting the p75 receptor (Pehar et al., 2004). Our in vivo data confirm that astrocytes from SOD1G93A mice express higher amounts of Ngf than non-transgenic astrocytes from the presymptomatic stage of disease. In addition, we have demonstrated that SOD1G93A astrocytes exhibit the same Ngf overproduction in vitro when cultured with normal motoneurons, without being activated by high levels of Lipopolysaccharide (LPS) or peroxynitrite (Pehar et al., 2004), and that this is accompanied by overexpression of p75 and its pro-apoptotic-associated protein, Ngfrap1, by non-transgenic motoneurons. Remarkably, the same transcriptional changes were found in motoneurons from SOD1G93A mice (Ferraiuolo et al., 2007), suggesting that the expression of mutant SOD1 by astrocytes can cause, in normal motoneurons, the same alterations observed in motoneurons from mutant SOD1 transgenic mice. Our in vitro model succeeded in reproducing pathogenic mechanisms observed in vivo without over-stimulating the cell model and without obtaining readouts far from the physiological levels of Ngf and lactate detected in human CSF samples.
We have shown for the first time that the overall increase in total NGF previously reported (Pehar et al., 2004) is due to an increase in the pro-NGF fraction, while the mature form of NGF tends to decrease, resulting in a pro-NGF/mature NGF ratio 2-fold higher than that detected in control conditions. Moreover, p75 over-expression by non-transgenic motoneurons correlates with and seems to be modulated by, the increasing levels of pro-NGF and is reduced by NGF immunodepletion. This offers an explanation for why total NGF immunodepletion had opposite effects on non-transgenic motoneurons depending on whether they were cultured with SOD1G93A transgenic or non-transgenic astrocytes. In the first case, the medium was cleared of a harmful stimulus, i.e. the activation of the pro-NGF-p75 cascade. In non-transgenic co-cultures, motoneurons were deprived of a positive neurotrophic stimulus, as p75 is not highly expressed and mature NGF is likely to be signalling predominantly through the TrkA receptor. Consistent with this interpretation, p75 receptor inhibition did not affect motoneuron viability in non-transgenic co-cultures, while it rescued motoneurons growing on SOD1G93A astrocytes. Our data suggest that pro-NGF overexpression can increase p75 receptor expression on motoneurons through a positive feedback mechanism, leading to increased cell death and p75 inhibition is related to a significant decrease in pro-NGF. How NGF regulates p75 receptor expression is still not clear (Carter et al., 1996), but it is noteworthy that the p75 promoter has a binding site for NfkB (http://www.sabiosciences.com/), which is a downstream effector of p75 activation (Carter et al., 1996).
Increased expression of pro-NGF has been reported to cause neuronal degeneration following CNS injury (Harrington et al., 2004) as well as motoneuron death in response to peroxynitrite (Domeniconi et al., 2007). The present study shows that astrocytes expressing mutant SOD1 per se do not express more pro-NGF than normal astrocytes, but the presence of normal motoneurons is sufficient to trigger this dysregulation. The presence of either normal or mutant SOD1 expressing motoneurons seems to elicit a dysregulated response from mSOD1-expressing astrocytes. The question is what factor(s) or molecule(s) expressed by motoneurons triggers the dysregulation. We tested the hypothesis that glutamate could be responsible for the altered astrocytic response (Magistretti et al., 1994; Bruno and Cuello, 2006). Our in vitro data confirmed that both lactate and NGF release increase when astrocytes are stimulated with glutamate for 14 days; however, over this period mutant SOD1 astrocytes did not display the alteration that they display when cultured with motoneurons. Interestingly, the levels of lactate released in the media following glutamate exposure are significantly lower than the levels achieved when motoneurons are in the culture, while NGF levels are lower. Our results support the hypothesis that glutamate could be involved in the astrocytic dysregulated response as a regulator of both lactate and NGF release, but we have to conclude that glutamate on its own is not responsible for the dysregulation observed in vitro. Whether glutamate acts in synergy with other factors secreted by motoneurons or other stimuli are involved in triggering astrocytic toxicity has yet to be determined. Moreover, although NGF and lactate have a common regulator, our data are unable to show a direct interaction between the pro-NGF/p75 pathway and lactate release. Lactate treatment led to a decrease in p75 expression on motoneurons, but was not related to a shift in the balance of pro-NGF/NGF, although it completely rescued motoneuron survival. The mild, but significant, improvement in p75 expression levels could be due to a general neuroprotective effect of lactate rather than a direct interaction between the lactate and the pro-NGF/p75 pathways. In addition, anti-p75 treatment did not have any effect on lactate levels. In summary, our results indicate that the two pathways, although both dysregulated, do not interact directly, but they contribute to motoneuron degeneration through different unrelated mechanisms. However, in vivo studies are required to fully understand the neuroprotective efficacy of intervening to correct the dysregulation of these two pathways over the time course of the disease in ALS. We also investigated the possibility that Fgf1 could be the factor responsible for pro-NGF (Cassina et al., 2005) and lactate dysregulation. Although both transgenic and non-transgenic astrocytes secrete comparable levels of Fgf1 in the media when in co-culture with non-transgenic motoneurons, our data indicate that Fgfr1 activation is linked to pro-NGF production. However, Fgfr1 inhibition does not seem to be a valid option as a neuroprotective strategy, since PD166866 led to massive motoneuron death in both transgenic and non-transgenic co-cultures. Our data on motoneuron survival are opposite to the data previously published (Cassina et al., 2005), but the experimental differences are likely to account for these contrasting outcomes. In our study, Fgfr1 inhibitor was added when motoneurons were already in the co-culture system, in contrast to the previous report, where astrocytes had been preconditioned with the inhibitor or high doses of Fgf1 (in the order of nanogram/millilitre) and motoneurons were plated and counted 72 h later. Our data show that an altered response to a physiological Fgf1 increase, rather than an abnormal increase in Fgf1, could be responsible for the change observed in pro-NGF/NGF ratio. The lack of changes in lactate secretion provides further confirmation that the dysregulation in NGF and lactate pathways are caused by different stimuli.
We consider our data of high relevance to human ALS. We have demonstrated for the first time increased levels of pro-NGF in the CSF of patients with ALS at the time of diagnosis. Previous studies detected increased expression in ALS spinal cord of matrix metalloproteinase 9 (MMP-9) (Niebroj-Dobosz et al., 2010), which is one of the enzymes degrading mature NGF in the extracellular space. These findings are consistent with our data indicating that the pro-NGF/mature NGF ratio is unbalanced in disease, with increased expression of the parent molecule pro-NGF. In addition, p75 messenger RNA over-expression in the spinal cord of patients with ALS indicates that this pathway may play a role during the disease course. One recent study has reported that pro-NGF induces upregulation of PTEN (phosphatase and tensin homolog deleted on chromosome 10) via p75, leading to cell death (Song et al., 2010). Interestingly, we have demonstrated that motoneurons that survive the disease process in human SOD1-related ALS appear to successfully downregulate the PTEN signalling pathway (Kirby et al., 2011).
In this scenario, correcting the imbalance between pro-NGF and mature NGF could be beneficial in modifying the disease course of ALS by blocking the detrimental effects of pro-NGF signalling through the p75 receptor and by increasing the neurotrophic potential of mature NGF (Davis-Lopez de Carrizosa et al., 2010), while therapies aiming to block the binding of both pro-NGF and mature NGF to their receptors are likely to be unsuccessful. One possible approach is inhibition of the p75 signalling cascade as shown in the in vitro experiments reported here, where an antibody was used to block p75 receptor activation. Although we have demonstrated that this strategy is successful in vitro, determining the right approach in vivo is of paramount importance in order to achieve neuroprotection of motoneurons within the CNS (Turner et al., 2004). Another possible approach would be to promote pro-NGF cleavage in order to shift the balance between its immature and mature form, re-establishing the ratio prevailing under physiological conditions.
In agreement with the findings of other groups showing that astrocytes expressing SOD1G93A are toxic to normal motoneurons from mice and motoneurons derived from human embryonic stem cells (Di Giorgio et al., 2007, 2008; Nagai et al., 2007), we also detected increased cell death when non-transgenic motoneurons were plated onto SOD1G93A astrocytes. These previous reports concluded that a soluble toxic factor was responsible for the increased motoneuronal death occurring in the mixed co-cultures, but were unable to identify this factor. Our findings add significantly to previous reports by demonstrating that pro-NGF is one toxic factor released by SOD1G93A astrocytes, which exerts a detrimental effect on motoneuron survival. Significantly however, there is an additional failure of mutant SOD1 astrocytes to provide the metabolic support required by motoneurons. The finding that lactate supplementation can completely reverse the observed toxicity is of considerable interest, particularly as metabolic dysregulation seems to be present in the early stages of disease both in the mutant SOD1 mouse model (Dupuis et al., 2004; Ferraiuolo et al., 2007) and in human ALS (Desport et al., 2000, 2005). Dietary interventions with the potential to ameliorate the motoneuron energy deficit have already been shown to be of benefit in mouse models of ALS (Zhao et al., 2006; Patel et al., 2010) and are being considered as a potential therapeutic approach in the human disease.
This study supports the hypothesis that motoneuron degeneration in SOD1-related ALS is a ‘non-cell-autonomous’ process. However, motoneurons themselves stimulate the pathophysiological properties in neighbouring astrocytes. Moreover, SOD1G93A transgenic astrocytes can also alter the expression of key molecules in normal motoneurons, rendering them more vulnerable to injury and cell death. Mouse models expressing mutant SOD1 selectively in astrocytes (Gong et al., 2000), however, failed to show motoneuron degeneration. The discrepancy between this in vivo model and our findings could be due to a number of factors, the first being the expression levels of transgene reached in the mouse model. Variable transgene expression levels have resulted in differing results when mutant SOD1 is expressed selectively in motoneurons (Pramatarova et al., 2001; Lino et al., 2002; Jaarsma et al., 2008). Moreover, although the in vitro system can clarify how single mechanisms operate, it does not allow prediction of the contribution of other cell types present in the spinal cord. It is possible that other non-neuronal cells, when not expressing mutant SOD1, can support motoneurons and protect them from degeneration.
Taken together, the data reported here confirm that alteration in several properties of astrocytes are likely to play a crucial role in the propagation of motoneuron injury in SOD1-related ALS and indicate that manipulation of the energy supply to motoneurons as well as inhibition of p75 receptor signalling may represent valuable approaches to ameliorate disease progression in ALS.
Supplementary material
Supplementary material is available at Brain online.
Funding
Wellcome Trust Programme award (GR069388) (to P.J.S.); Peake Fellowship award (to L.F.).
Supplementary Material
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- NGF
nerve growth factor
- Slc16a4
solute carrier 16a4
- SOD1
superoxide dismutase 1
References
- Bierl MA, Jones EE, Crutcher KA, Isaacson LG. ‘Mature’ nerve growth factor is a minor species in most peripheral tissues. Neurosci Lett. 2005;380:133–7. doi: 10.1016/j.neulet.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Bilsland LG, Nirmalananthan N, Yip J, Greensmith L, Duchen MR. Expression of mutant SOD1 in astrocytes induces functional deficits in motoneuron mitochondria. J Neurochem. 2008;107:1271–83. doi: 10.1111/j.1471-4159.2008.05699.x. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–92. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. The neurotrophin receptors, trkB and p75, differentially regulate motor axonal regeneration. J Neurobiol. 2001;49:314–25. doi: 10.1002/neu.10013. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci USA. 2006;103:6735–40. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BD, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm-Matthaei R, Baeuerle PA, et al. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–5. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- Cassina P, Pehar M, Vargas MR, Castellanos R, Barbeito AG, Estevez AG, et al. Astrocyte activation by fibroblast growth factor-1 and motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2005;93:38–46. doi: 10.1111/j.1471-4159.2004.02984.x. [DOI] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–7. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- Davis-Lopez de Carrizosa MA, Morado-Diaz CJ, Morcuende S, de la Cruz RR, Pastor AM. Nerve growth factor regulates the firing patterns and synaptic composition of motoneurons. J Neurosci. 2010;30:8308–19. doi: 10.1523/JNEUROSCI.0719-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5:1131–6. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- Desport JC, Preux PM, Truong CT, Courat L, Vallat JM, Couratier P. Nutritional assessment and survival in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:91–6. doi: 10.1080/14660820050515386. [DOI] [PubMed] [Google Scholar]
- Desport JC, Torny F, Lacoste M, Preux PM, Couratier P. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neurodegener Dis. 2005;2:202–7. doi: 10.1159/000089626. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–48. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–14. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Hempstead BL, Chao MV. Pro-NGF secreted by astrocytes promotes motor neuron cell death. Mol Cell Neurosci. 2007;34:271–9. doi: 10.1016/j.mcn.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci U S A. 2004;101:11159–64. doi: 10.1073/pnas.0402026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiuolo L, De Bono JP, Heath PR, Holden H, Kasher P, Channon KM, et al. Transcriptional response of the neuromuscular system to exercise training and potential implications for ALS. J Neurochem. 2009;109:1714–24. doi: 10.1111/j.1471-4159.2009.06080.x. [DOI] [PubMed] [Google Scholar]
- Ferraiuolo L, Heath PR, Holden H, Kasher P, Kirby J, Shaw PJ. Microarray analysis of the cellular pathways involved in the adaptation to and progression of motor neuron injury in the SOD1 G93A mouse model of familial ALS. J Neurosci. 2007;27:9201–19. doi: 10.1523/JNEUROSCI.1470-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J Neurosci. 2000;20:660–5. doi: 10.1523/JNEUROSCI.20-02-00660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, et al. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci USA. 2004;101:6226–30. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayworth CR, Gonzalez-Lima F. Pre-symptomatic detection of chronic motor deficits and genotype prediction in congenic B6.SOD1(G93A) ALS mouse model. Neuroscience. 2009;164:975–85. doi: 10.1016/j.neuroscience.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28:154–64. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Bloch-Gallego E, Camu W. Purified embryonic motoneurons. Oxford: IRL Press; 1995. [Google Scholar]
- Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28:2075–88. doi: 10.1523/JNEUROSCI.5258-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J, Ning K, Ferraiuolo L, Heath PR, Ismail A, Kuo SW, et al. Phosphatase and tensin homologue/protein kinase B pathway linked to motor neuron survival in human superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain. 2011;134:506–17. doi: 10.1093/brain/awq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KC, Tyler CM, Lim ST, Giuliano R, Federoff HJ. Proteolytic processing of proNGF is necessary for mature NGF regulated secretion from neurons. Biochem Biophys Res Commun. 2007;361:599–604. doi: 10.1016/j.bbrc.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Lino MM, Schneider C, Caroni P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci. 2002;22:4825–32. doi: 10.1523/JNEUROSCI.22-12-04825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Sorg O, Naichen Y, Pellerin L, de Rham S, Martin JL. Regulation of astrocyte energy metabolism by neurotransmitters. Ren Physiol Biochem. 1994;17:168–71. doi: 10.1159/000173810. [DOI] [PubMed] [Google Scholar]
- Moubarak RS, Sole C, Pascual M, Gutierrez H, Llovera M, Perez-Garcia MJ, et al. The death receptor antagonist FLIP-L interacts with Trk and is necessary for neurite outgrowth induced by neurotrophins. J Neurosci. 2010;30:6094–105. doi: 10.1523/JNEUROSCI.0537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai J, Suvant P, Sato TA. Nerve growth factor-dependent regulation of NADE-induced apoptosis. Vitam Horm. 2003;66:385–402. doi: 10.1016/s0083-6729(03)01011-2. [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–22. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebroj-Dobosz I, Janik P, Sokolowska B, Kwiecinski H. Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Eur J Neurol. 2010;17:226–31. doi: 10.1111/j.1468-1331.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- Patel BP, Safdar A, Raha S, Tarnopolsky MA, Hamadeh MJ. Caloric restriction shortens lifespan through an increase in lipid peroxidation, inflammation and apoptosis in the G93A mouse, an animal model of ALS. PLoS One. 2010;5:e9386. doi: 10.1371/journal.pone.0009386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehar M, Cassina P, Vargas MR, Castellanos R, Viera L, Beckman JS, et al. Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2004;89:464–73. doi: 10.1111/j.1471-4159.2004.02357.x. [DOI] [PubMed] [Google Scholar]
- Pehar M, Vargas MR, Robinson KM, Cassina P, Diaz-Amarilla PJ, Hagen TM, et al. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J Neurosci. 2007;27:7777–85. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–62. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci. 2001;21:3369–74. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–9. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- Song W, Volosin M, Cragnolini AB, Hempstead BL, Friedman WJ. ProNGF Induces PTEN via p75NTR to Suppress Trk-Mediated Survival Signaling in Brain Neurons. J Neurosci. 2010;30:15608–15. doi: 10.1523/JNEUROSCI.2581-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Murray SS, Piccenna LG, Lopes EC, Kilpatrick TJ, Cheema SS. Effect of p75 neurotrophin receptor antagonist on disease progression in transgenic amyotrophic lateral sclerosis mice. J Neurosci Res. 2004;78:193–9. doi: 10.1002/jnr.20256. [DOI] [PubMed] [Google Scholar]
- Van Gelder RN, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci USA. 1990;87:1663–7. doi: 10.1073/pnas.87.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B. Preparation and coculture of neurons and glial cells. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0207s32. Chapter 2: Unit 2 7. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–3. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Lee SK, Sato TA, Koh JY. Co-induction of p75(NTR) and the associated death executor NADE in degenerating hippocampal neurons after kainate-induced seizures in the rat. Neurosci Lett. 2003;347:126–30. doi: 10.1016/s0304-3940(03)00656-6. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, et al. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.