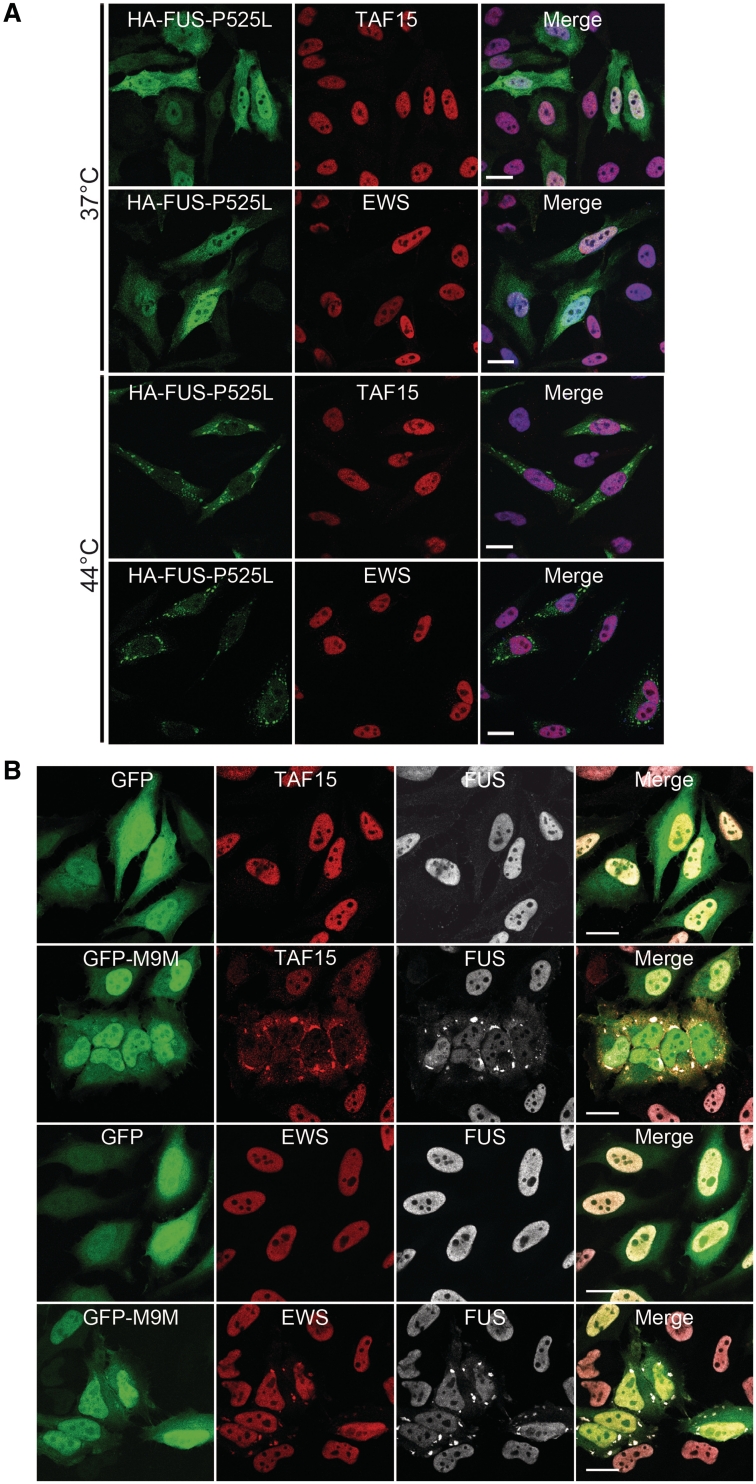
Figure 7.
Analysis of FET proteins in cell culture systems. (A) Cytoplasmically mislocalized mutant FUS does not sequester TAF15 or EWS into stress granules upon heat shock. HeLa cells transiently transfected with haemagglutinin-tagged human FUS with the P525L mutation (HA-FUS-P525L) were left untreated (37°C, top) or subjected to heat shock (1 h at 44°C, bottom) 24 h after transfection. Cells were stained with antibodies against haemagglutinin (green) and EWS (red) or TAF15 (red) and analysed by confocal microscopy. Under control conditions, HA-FUS-P525L is diffusely distributed in the cytoplasm, and endogenous TAF15 and EWS is localized in the nucleus. Upon heat shock, HA-FUS-P525L is recruited into cytoplasmic stress granules, while TAF15 and EWS remain predominantly nuclear and are not entrapped into FUS-positive stress granules. Scale bar = 20 µm. (B) Inhibition of the Transportin pathway leads to cytoplasmic mislocalization of TAF15, EWS and FUS into stress granules. The Transportin-specific peptide inhibitor M9M fused to green fluorescent protein (GFP-M9M, green) or GFP alone was expressed in HeLa cells for 24 h. Cells were stained with antibodies against TAF15, EWS (both shown in red) and FUS (white) and were analysed using confocal microscopy. Upon inhibition of Transportin-mediated nuclear import by the GFP-M9M peptide, TAF15 and EWS are recruited into cytoplasmic stress granules, where they co-localize with FUS. Note that EWS shows only mild cytoplasmic mislocalization, while FUS and especially TAF15 show a marked cytoplasmic redistribution with a nuclear depletion of these proteins. Scale bar = 20 µm.