Figure 1.

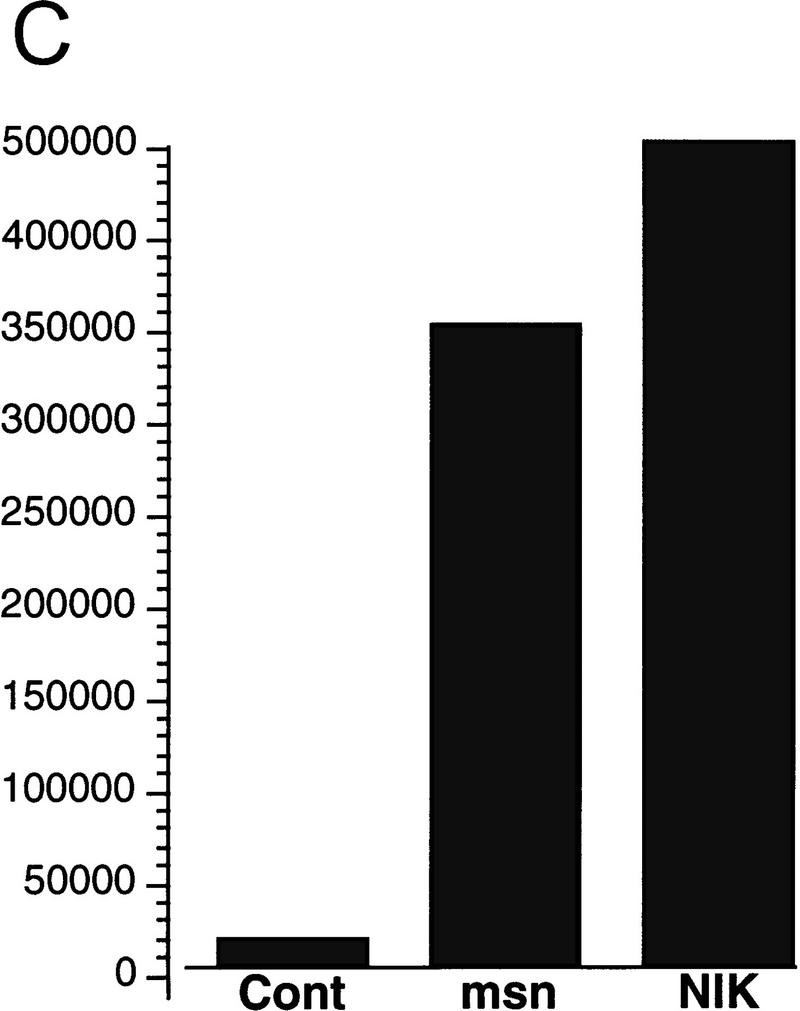
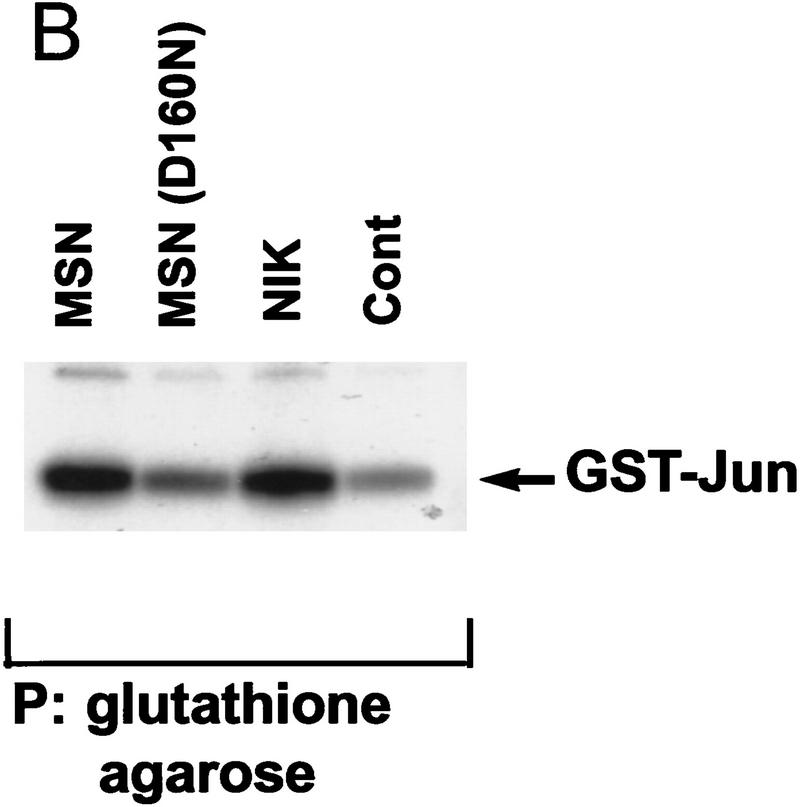
(A) Amino acid alignment of the deduced amino acid sequence of msn, NIK, and mig-15. Identical amino acids are shown in black. The amino-terminal kinase domain and the carboxy-terminal regulatory domain show a high degree of identity between the three proteins. (B,C) Activation of JNK by msn and NIK. (B) msn, NIK, and msn (D160N), which is a kinase inactive form of msn with asparagine substituted for aspartic acid at position 160, were transfected into 293 cells together with 1 μg of GST-tagged JNK. JNK activity in the transfected cell lysates was then determined as described in Materials and Methods. Equal amounts of GST–JNK were precipitated between samples (data not shown). (C) msn (0.2 μg) was cotransfected into 293 cells together with 10 ng of a plasmid expressing a fusion protein consisting of ATF2 and GAL4 DNA binding domain and 5 μg of a plasmid espressing a GAL4–luciferase reporter (Su et al. 1997). Transfection efficiency was assessed by cotransfecting 1 μg of a plasmid expressing β-galactosidase under the control of an SV40 promoter. Luciferase activity is expressed in arbitrary units after being standardized to β-galactosidase activity.