Abstract
Brassica juncea is promising for metal phytoremediation, but little is known about the functional role of most metal transporters in this plant. The functional characterization of two B. juncea cation-efflux family proteins BjCET3 and BjCET4 is reported here. The two proteins are closely related to each other in amino acid sequence, and are members of Group III of the cation-efflux transporters. Heterologous expression of BjCET3 and BjCET4 in yeast confirmed their functions in exporting Zn, and possibly Cd, Co, and Ni. Yeast transformed with BjCET4 showed higher metal resistance than did BjCET3 transformed. The two BjCET–GFP fusion proteins were localized to the plasma membrane in the roots when expressed in tobacco, and significantly enhanced the plants’ Cd tolerance ability. Under Cd stress, tobacco plants transformed with BjCET3 accumulated significant amounts of Cd in shoots, while maintaining similar shoot biomass production with vector-control subjects. Transformed BjCET4 tobacco plants showed significantly enhanced shoot biomass production with markedly decreased shoot Cd content. The two transporter genes have a lower basal transcript expression in B. juncea seedling tissues when grown in normal conditions than under metal-stress, however, their transcripts levels could be substantially increased by Zn, Cd, NaCl or PEG, suggesting that BjCET3 and BjCET4 may play roles in several stress conditions, roles which appear to be different from those of previous characterized cation-efflux transporters, for example, AtMTP1, BjCET2, and BjMTP1.
Keywords: B. juncea L., cation-efflux transporter, heavy metal stress, heavy metal tolerance, osmotic stress, transgenic plant
Introduction
Transition metals such as Zn and Cu are essential for many processes in plants, but are toxic when they accumulate to high levels (Godbold and Hüttermann, 1985; Dietz et al., 1999; Nies, 1999). However, a few plants have special mechanisms for metal-ion homeostasis and detoxification, and allow normally toxic levels of metals accumulate in their tissues. For example, two members of the Brassicaceae family, Thlaspi caerulescens and Thlaspi goesingense, are able to grow in soil containing Cd2+, Ni2+, Zn2+, and accumulate 100–1000 times higher concentrations of such heavy metals in the shoots than typical species, without any symptoms of toxicity (Persans et al., 2001; Rigola et al., 2006). In recent years, it has been learned that metal membrane transport systems probably play a critical role in this metal hyperaccumlation (Hall and Williams, 2003; Krämer et al., 2007; Milner and Kochian, 2008; Kim et al., 2009)
With the application of molecular and genetic techniques, a number of gene families involved in metal transport and homeostasis have been identified. These include: the natural resistance-associated macrophage protein (NRAMP) family with members encoding multispecific metal transporters, described in Fe and Mn homeostasis (Curie et al., 2000; Thomine et al., 2000); the cation exchanger (CAX) family involved in Ca2+/H+, Na+/H+, or Mn2+/H+ antiport (Hirschi et al., 2000); the Zrt, Irt-like protein (ZIP) family involved in transporting a variety of divalent cations including Zn2+, Fe2+, Mn2+, and Cd2+ (Guerino, 2000); and the cation efflux (CE) family including members from Arabidopsis thaliana involved in Zn homeostasis (van der Zaal et al., 1999). Proteins belonging to the cation-efflux family (also known as the cation diffusion facilitator family) were first described by Nies and Silver in 1995 (Nies and Silver, 1995), and have been implicated in conferring Zn2+, Cd2+, Co2+ or Ni2+ tolerance in a range of organisms. Recently a distinct phylogenetic group of cation-efflux proteins (ShMTP8 and AtMTP11) were shown to be specific for Mn export from the cytoplasm (Delhaize et al., 2003, 2007; Peiter et al., 2007). Most cation-efflux proteins are transporters that either sequester metal ions within cells or export metal ions out of cells (Paulsen and Saier, 1997). Interest in cation-efflux proteins has greatly increased because of evidence that cation-efflux proteins may be useful in phytoremediation, food fortification, and treating human disease (Ford, 2004; Krämer et al., 2007). Although there is evidence that overexpression of a cation-efflux member in plants can decrease metal toxicity and enhance metal accumulation, up to now little is known about the function and mechanisms of cation-efflux transporters.
The A. thaliana genome encodes 12 putative cation-efflux proteins (AtMTP1 to AtMTP12), which can be divided into three distinct groups (Blaudez et al., 2003). Group I (AtMTP8 to AtMTP11 and AtMTP6, AtMTP7) and Group II (AtMTP5 and AtMTP12) proteins contain only a subset of cation-efflux features, described as having 15 (AtMTP12) or four to five predicted transmembrane domains, only part of the N-terminal signal sequence, or lacking the His-rich domain (Paulsen and Saier, 1997). Group III (AtMTP1 to AtMTP4) contains all the basic cation-efflux features (Paulsen and Saier, 1997). Most of the cation-efflux proteins are involved in metal tolerance. For example, the ZAT gene of Arabidopsis (also known as AtMTP1) confers Zn2+ tolerance when over-expressed in plants (van der Zaal et al., 1999). The increased tolerance was thought to be attributable to internal sequestration of Zn2+, which was confirmed later by the subcellular location of the AtMTP1–GFP fusion protein to the vacuolar membrane in root and leaf of Arabidopsis cells (Kobae et al., 2004; Desbrosses-Fonrouge et al., 2005). Homologues of AtMTP1 have been found in metal hyperaccumulators including TgMTP1 in Thlaspi goesingense (Persans et al., 2001), TcMTP1 in Thlaspi caerulescens (Assucao et al., 2001), and AhMTP1 in Arabidopsis halleri (Dräger et al., 2004). All these MTP1 proteins showed a constitutively higher shoot expression in the hyperaccumulators than in non-accumulators, a feature that is therefore supposed to be a key contribution to metal hyperaccumulation, a hypothesis supported from recent molecular genetic evidence (Filatov et al., 2006; Milner and Kochian, 2008). Based on the localization of MTP1–GFP fusion proteins in yeast or plant cells, AhMTP1 was found in the vacuolar membrane (Dräger et al., 2004), whereas TgMTP1 was localized to both the cytoplasmic membrane and vacuolar membrane (Kim et al., 2004; Gustin et al., 2009). MTP1 proteins have been shown to confer zinc tolerance to Saccharomyces cerevisiae mutants defective in one or both of the cation-efflux transporter COT1 and ZRC1 that transport zinc ions into the vacuole (Persans et al., 2001; Bloss et al., 2002; Blaudez et al., 2003; Dräger et al., 2004; Kim et al., 2004; Desbrosses-Fonrouge et al., 2005; Arrivault et al., 2006).
Phytoremediation is a major concern (Chaney, 1983). Because of its multi-metal (such as Zn, Cr, Cu, Cd, Se, and Pb) accumulating ability and relatively high biomass production with fast growth rate and susceptibility to genetic manipulation, B. juncea has been taken as a potential metal hyperaccumlator for practical use in phytoremediation (Salt et al., 1995, 1997, 1998). A molecular understanding of B. juncea cation-efflux transporters, and their role in metal homeostasis, has important biotechnological applications, such as the design of metal accumulating plants to help combat metal-deficient and metal-contaminated soil conditions, as well increasing the metal ion content of plant foods. Four novel cation-efflux family transporters (BjCET1–BjCET4) have previously been cloned and recombinantly expressed from Cd treated seedlings of B. juncea (Lang et al., 2005a), and some functional properties of BjCET2 have been reported (Xu et al., 2009). As both BjCET3 and BjCET4 show high sequence similarity with each other and are in Group III of the CE family, it is hypothesized that BjCET3 and BjCET4 may perform similar functions of metal ion homeostasis in B. juncea, especially for Zn2+. The molecular and functional characteriztion of BjCET3 and BjCET4, which have some different functional properties from BjCET2, are described here. The proteins were expressed in mutant yeast and tobacco in medium oversupplied with metal to investigate their role in metal homeostasis and metal tolerance and accumulation. CET–GFP fusion proteins were used to determine their subcellular location in plant cells and semi-quantitative RT-PCR was used to check their mRNA levels in B. juncea under normal and adverse stress conditions.
Materials and methods
Plant materials and growth conditions
Seeds of B. juncea were a gift of the North Central Regional Plant Introduction Station (NCRPIS) of the United States National Plant Germplasm System (NPGS). Nicotiana tabacum cv. W38 was grown routinely in a greenhouse and B. juncea was grown as previous described by Lang et al. (2005b). All plants were grown under a 16 h light and 8 h dark cycle at 25 °C.
Cloning of BjCET genes
Using a PCR-enriched cDNA pooling method, four cation-efflux transporter genes were cloned from Cd-treated seedlings of B. juncea, which include BjCET3 and BjCET4 (Lang et al., 2005a).
Stress and semi-quantitative RT-PCR (sqRT-PCR)
B. juncea seedlings were hydroponically grown in 4.0 l of aerated 0.5× Hoagland's medium, changed every third day. Up to the 5–6 leaf stage, the seedlings were subjected to stress for 4, 8, 12, and 24 h or only for 12 h, by transferring to the aerated liquid medium with or without salt (250 mM NaCl), PEG (20% w/v PEG6000) or heavy metals (200 μM CdCl2 or 1000 μM ZnCl2). After each treatment, seedlings were washed with distilled water and the roots, stems, and leaves were separated and snap frozen in liquid nitrogen immediately. Total RNA was extracted using the RNAiso Plus reagent (TaKaRa), following the manufacturer's protocol and using the DNase digestion step to reduce DNA contamination. cDNA was reverse transcribed using PrimeScript™ 1st Strand cDNA Synthesis Kit (TaKaRa) by using 1 μg total RNA as the template. The B. juncea actin gene was used as an internal control for the relative quantification of transcript levels. The primer sequences were as follows: 5′-CTGTTACCACTCGTCACCATC-3′ and 5′-ACCTTGTTGAGCACCATATCTG-3′ for BjCET3 to amplify a 499 bp product, 5′-TGATGAAGAACAGAGAACAACAAC-3′ and 5′-CGCAGCAACAAGGAACATAAG-3′ for BjCET4 to amplify a 445 bp product, and 5′-AAGATCTGGCATC ACACTTTC-3′ and 5′-TAGTCAACAGCAACAAAGGAG-3′ for BjACTIN to amplify a 529 bp product, respectively. Three biological replicate samples were used for all analyses, and various cycle numbers were applied to ensure that, under the chosen PCR conditions, the products (both actin and the specific BjCET) were in the linear amplification range (data not shown). PCR band intensities were measured using Carestream MI software.
Yeast experiments for the metal tolerance assay
For yeast expression, the BjCET cDNAs were cloned into the pYES2 vector (Invitrogen), and transformed into three S. cerevisiae mutant strains (a kind gift from Professor DH Nies) by means of a chemical method (Gietz and Woods, 1994): YK40 (ura3-52 his3-200, Δcot1, MATα), YK41(ura3-52 his3-200, Δzrc1, MATα), and YK44 (ura3-52 his3-200, Δzrc1, Δcot1, MATα). The transformed yeast cells were selected on SMM minimal medium lacking uracil [SMM-uracil, 6.7 g yeast nitrogen base without amino acids, 20 mg histidine, 2% (w/v) glucose, 2% (w/v) agar l−1] and confirmed by PCR amplification of BjCET inserts. The metal tolerance assay was performed according to Persans et al. (2001). Briefly, yeast strains were grown to 1.5 OD600 in 10 ml of SMM–uracil liquid medium. Using 1 ml of yeast culture to inoculate 50 ml of SMM–uracil medium [replacing glucose with 4% (w/v) galactose and adding 1.5% (w/v) top agar l−1], the solution was mixed well and then poured into 90×15 mm plastic sterile dishes and allowed to cool. Five 4 mm sterile paper filters were placed at regular intervals on the surface of the top agar for each dish, and solutions containing various metal ions were spotted on to the discs. The plates were cultured in a 30 °C incubator for 3–4 d.
Yeast experiments for the metal accumulation assay
To determine the efflux transport activity of BjCET proteins, Zn accumulation in yeast cells was tested after an oversupply of Zn. Overnight cultures of single zrc1 yeast colonies transformed with BjCET3, BjCET4, and a empty vector were added to 200 ml SMM–uracil liquid medium to OD600=0.2. The cultures were then incubated to reach OD600=0.4. ZnCl2 (125 μM final concentration) was added, and the culture was incubated for another 4 h. Yeast cells were collected by centrifugation and first washed with 20 mM EDTA and then washed with sterilized deionized water 3–4 times. The samples were dried in an oven and then weighed. The Zn accumulation in samples was determined by the inductively coupled plasma-atomic emission spectrophotometry (ICP-MS) method.
Generation of transgenic tobacco expressing BjCET:GFP fusion proteins and confocal microscopy
The pBI121-GFP vector (a kind gift from Dr Jim Haseloff, Cambridge, England) contains the cauliflower mosaic virus 35S promoter and an NOS (nopaline synthase) terminator, and an nptII cassette was used for selection in plants under the control of the nos promoter and terminator. The BjCETs cDNAs were inserted into the XbaI and BamHI sites of the pBI121–GFP vector, and fused with GFP under the control of 35S promoter. The expression vector pBI121–BjCET–GFP and the empty vector pBI121–GFP were introduced into Agrobacterium tumefaciens (EHA105 strain) by the freeze–thaw method (Walkerpeach and Velten, 1994). About 1 cm2 regions of tobacco leaves were excised and co-cultured with Agrobacterium by the leaf disc-infection method (Horsch et al., 1985). Transformants were selected on MS medium supplemented with 200 mg l−1 of kanamycin and 250 mg l−1 of Cef. Putative transgenic plantlets were screened under a fluorescence microscope (485 nm) to assess green fluorescence and checked with PCR using the forward primer 5′-CCCGGATCCAAGGAGATATAAC-3′ and the reverse primer 5′-CCCGAGCTCTTATTTGTATAGTTCATCC-3′ to detect about 800 bp GFP fragments by using the transgenic plants’ genomic DNA as a template. Positive plantlets were then transferred to soil and allowed to flower and set seed in a greenhouse. The T0 transgenic plantlets were amplifed by using a tissue culture method. The differentiation and regeneration medium MT0 is MS supplemented with 1 mg l−1 6-BA, 0.1 mg l−1 NAA , 200 mg l−1 kanamycin, 250 mg l−1 Cef ,and 8 g l−1 agar, pH 5.8. The rooting medium MT1 is 1/2 MS supplemented with 0.1 mg l−1 NAA, 200 mg l−1 kanamycin, 250 mg l−1 Cef, and 8 g l−1 agar, pH 5.8. The tissue culture amplified T0 transgenic plants were used for the following heavy metal tolerance and accumulation assay.
Heavy metal accumulation and tolerance assay
About 1 cm2 regions of leaves were excised from WT and T0 transgenic tobaccos and placed in MT0 medium in which kanamycin had been replaced with 200 μM CdCl2. Each bottle contained 10 leaf explants and was cultured in an incubator at 25 °C with 16 h d−1 of light. After 35 d, the differentiated seedlings were counted. In the meantime, the 2–3 cm regenerated seedlings of WT and transgenic plants from regular medium were planted onto MT1 rooting medium containing 200 μM CdCl2. After 15 d, the rooting results were observed and, after 30 d, the plants were harvested and the plant growth index was measured. For determining Cd content, plant samples were firstly washed 5–7 times with distilled water, then dried overnight at 70 °C, weighed, and finely ground. Samples (0.1 g) were digested with 2 ml nitric acid by heating discontinuously in microwave for 2 min. After the solution was cooled, it was transferred to a 50 ml flask and made up to volume with deionized water before being analysed by the ICP-MS method.
Statistical analysis
Data were analysed using a one-way ANOVA test to our endpoints, followed by a Tukey test when data were statistically different (P <0.05) (Zar, 1996). Values are hte average number of each genotype (arithmetic mean ±SD of n=3 independent experiments).
Results
Cloning and sequence analysis of B. juncea BjCET3 and BjCET4 cDNAs
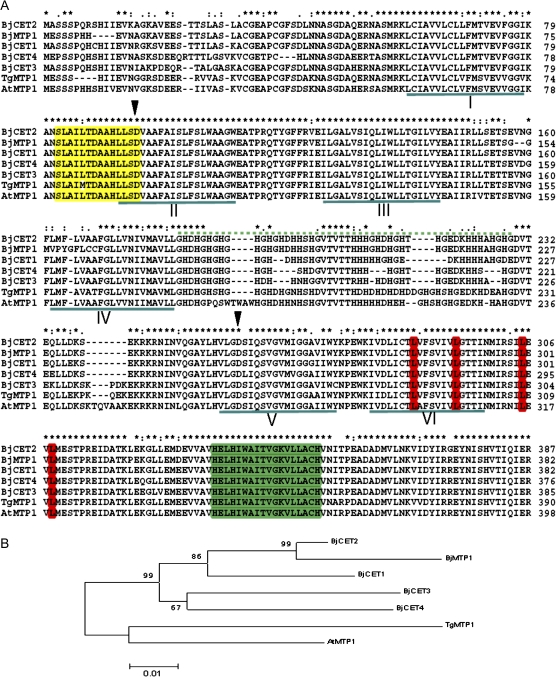
Lang et al. (2005a) isolated four previous unidentified full-length cation-efflux family cDNA sequences from Cd stressed B. juncea and named them BjCET1–BjCET4 (Fig. 1A). Muthukumar et al. (2007) cloned BjMTP1, another cation-efflux family member of B. juncea. cDNAs of BjCET3 and BjCET4 contained putative ORFs of 1155 and 1128 bp encoding 385 and 376 amino acid residues with predicted molecular masses of 42.4 and 41.4 kDa, respectively (see Supplementary Table S1 at JXB online). The deduced amino acid sequences showed strong similarities (pairwise identity of 81–89%) among the five cation-efflux proteins from B. juncea, and to the previously characterized CE proteins ZAT, AhCDF1-3, and TgMTP1 (76–82%) (Fig. 1B). BjCET3 and BjCET4, and BjCET2 and BjMTP1 are the most similar pairs in a phylogenetic tree analysis (Figs 1B, 2). BjCET3 and BjCET4 contain features of Group III cation-efflux proteins (Fig. 1A).
Fig. 1.
BjCET sequence analysis. (A) Alignments of predicted amino acid sequences for BjCET1, BjCET2, BjCET3, BjCET4, and BjMTP1 from B. juncea, TgMTP1 from T. goesingense and AtMTP1 from A. thaliana. Sequence alignment was performed by CLUSTALW. The following protein features have been highlighted: the six transmembrane domains (TMs) identified by TMHMM (version 2.0) (underlined in dark green); the CDF signature sequences (yellow); the histine-rich loop region (dashed dark green line); the highly conserved aspartate (filled inverted triangles)in domain II and V (Bloss et al., 2002); the C-terminal LZ motif (red) (Blaudez et al., 2003); and the C-terminal putative Zn binding site HD(E)XHXWXL(I)TX8H (dark green) (Dräger et al., 2004). (B) Phylogenetic analysis of the identified CE proetins from B. juncea. The Neighbor–Joining phylogenetic tree was constructed using MEGA4.1 after CLUSTALW alignment of the full-length amino acid sequences.
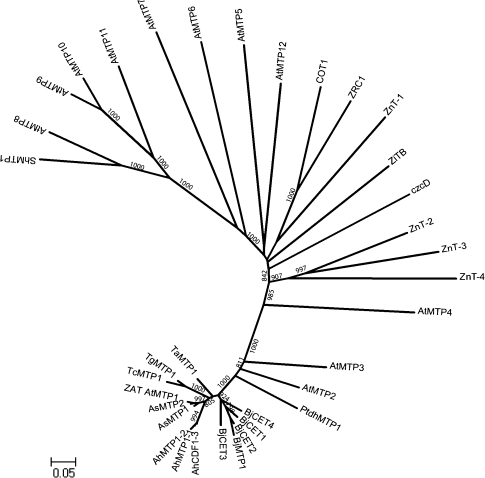
Fig. 2.
Phylogenetic analysis of the CE proteins from B. juncea and other genera. The Neighbor–Joining phylogenetic tree based on the protein alignment was created with MEGA 4.1. The sequences were aligned with Clustal X software, and the scale bar indicates an evolutionary distance of 0.05 amino acid substitution per site. The number at the nodes show bootstrap values (>800) after 1000 replicates. Accession numbers and species names of each gene are shown in Supplementary Table S2 at JXB online.
The predicted amino acid sequences of 32 cation-efflux genes from different species were analysed using Clustal X (see Supplementary Fig. S1 at JXB online). Cation-efflux members with common features from the same source mostly group together. Analysis through a phylogenetic tree (Figs 1B, 2), showed that the plant Group III cation-efflux proteins are more closely grouped than others (Blaudez et al., 2003). The phylogenetic tree also showed that all the cloned cation-efflux members with common features could be grouped based on species, for example, ZiTB and czcD, bacteria source; COT1 and ZRC1, yeast source. Almost all plant source cation-efflux members with common features are situated on one clade, and cation-efflux members from the same plant species are usually situated on one subclade, for example, Thlaspi source of TgMTP1 and TcMTP1, Arabidopsis source of AhMTP1-1, AhMTP1-2, and AhCDF1-3. The newly identified Brassica cation-efflux members BjCET1-4 and BjMTP1 are also situated on one subbranch, and BjCET3 and BjCET4 are grouped together in the third subclade, suggesting that the two cDNAs are more evolutionarily and functionally related (Figs 1B, 2). However, the Group III proteins AtMTP1–4 from A. thaliana showed more divergence compared with BjCETs. Montanini et al. (2007) classified the majority of CDF family members into three groups (Zn, Fe/Zn, and Mn) based on phylogenetic reconstruction, and each group prefered a special metal selectivity. Our new BjCET members fall into Zn-CDF group, which suggests they may transport zinc. Their other possible substrates are Co2+, Cd2+, and Ni2+.
BjCET3 and BjCET4 response to multiple abiotic stress in addition to Cd and Zn
The expression patterns of BjCET3 and BjCET4 genes in different tissues (roots, stems, and leaves) and under different growth conditions were examined by sqRT-PCR. Specific primers for each BjCET gene were designed and tested for amplifying only the target BjCET (data not shown). As B. juncea is a Cd accumulator and tolerant to high Cd concentration than normal plants (Salt et al., 1995), a little higher Cd concentration (200 μM CdCl2) was used to stress the B. juncea plantlets, but that concentration was not lethal to the plant during the 24 h and even longer times of treatment (see Supplementary Fig. S2 at JXB online).
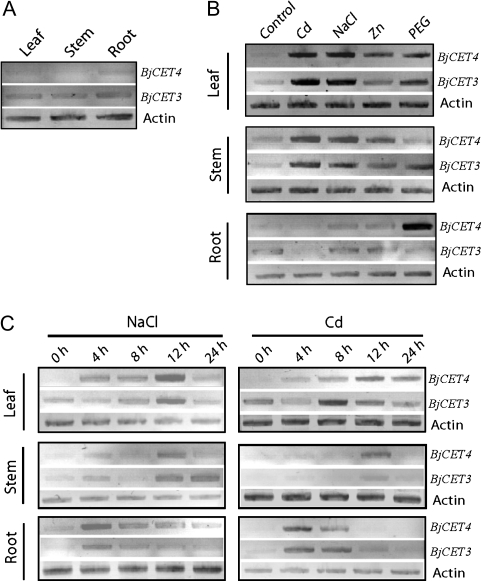
The basal mRNA levels of BjCET3 and BjCET4 in the roots, stems, and leaves of 5–6 leaf stage B. juncea seedlings grown in normal conditions were checked. The sqRT-PCR could hardly detect the BjCET4 transcript in the leaf and stem, and was only detected a little more in the root. The method showed a higher BjCET3 transcript level in all three tissues than for BjCET4 (Fig. 3A).
Fig. 3.
sqRT-PCR analysis of steady-state levels of BjCET3 and BjCET4 transcripts and their regulation by Zn, Cd, NaCl, and PEG in root, stem, and shoot tissues of B. juncea. (A) BjCET3 and BjCET4 mRNA expression level in tissues of the 5–6 leaf stage of B. juncea seedlings growing in normal conditions; (B) BjCET3 and BjCET4 expression level in tissues of the 5–6 leaf stage of B. juncea seedlings after 12 h treatment with 200 μM CdCl2, 1000 μM ZnCl2, 250 mM NaCl, and 20% w/v PEG6000, respectively; (C) BjCET3 and BjCET4 expression level in tissues of the 5–6 leaf stage of B. juncea seedlings after 4, 8, 12 , and 24 h treatment with 200 μM CdCl2 and 250 mM NaCl, respectively. 25 cycles were used for all PCR reactions, which were tested in the linear amplification range for each target gene.
A 12 h stress treatment was then made for the 5–6 leaf stage of B. juncea seedlings with 200 μM CdCl2 , 1000 μM ZnCl2, 250 mM NaCl, and 20% PEG 6000 (w/v), respectively, and the various stress effects on BjCET3 and BjCET4 transcript levels in different tissues was checked (Fig. 3B). In the root, it was found that the BjCET4 transcript level was increased 6.8-fold upon PEG treatment, and it showed a slight increase upon Zn and NaCl stress, but vitually no change upon Cd stress. The BjCET3 transcript level did not change much in response to Zn, NaCl, and PEG treatment, but showed some decrease in response to Cd treatment. In the stem, the BjCET4 transcript level showed 1.9-, 1.7-, and 2.1-fold increase in response to Cd, Zn, and NaCl stress, respectively, while nearly no change was seen in response to PEG treatment. However, in addition to the up-regulation upon Cd (3.9-fold) and NaCl (3.7-fold) stress, the BjCET3 transcript level increased 4.0-fold after 12 h of PEG treatment, and it also showed a minor increase with Zn stress. In the leaf, both BjCET3 and BjCET4 transcript levels were markedly increased with Cd (6.4-fold for BjCET3, 4.9-fold for BjCET4), NaCl (5.7-fold for BjCET3, 5.5-fold for BjCET4), and PEG (3.6-fold for BjCET3, 4.4-fold for BjCET4) treatments, while in response to the Zn treatment, the BjCET3 transcript level still showed a small (1.5-fold) increase, but BjCET4 showed a stronger (3.5-fold) increase.
Time-dependent changes in the expression of BjCET3 and BjCET4 in response to Cd and NaCl treatment were also examined (Fig. 3C). In the root, both BjCET3 and BjCET4 mRNA levels peaked at 4 h and then decreased during the following 8–24 h treatment. In the stem, the BjCET4 mRNA level peaked at 12 h upon Cd and NaCl treatment, then dropped and fell below the limit of detection at 24 h. The BjCET3 transcript level was also observed to peak at 12 h (3.2-fold increase) upon Cd treatment, then decreased. However, it showed a higher level at both 12 h (4.0-fold increase) and 24 h (4.5-fold increase) during the NaCl treatment. In the leaf, the BjCET4 mRNA level had increased at 4 h and peaked at 12 h with Cd and NaCl treatments. However, at 24 h, the mRNA level of BjCET4 was markedly reduced during the NaCl treatment, but continued to be higher in the Cd treatment. The BjCET3 expression peaked at 8 h and 12 h upon Cd and NaCl treatment, respectively, and then dropped to normal levels.
BjCET3 and BjCET4 confer a broad range of metal tolerance in yeast complementation assay
To investigate the substrate specificity of BjCET3 and BjCET4 and their functional properties, they were expressed in yeast mutants deficient in their orthologues COT1 and ZRC1. These mutants are hypersensitive to Co and Ni, or Cd and Zn.
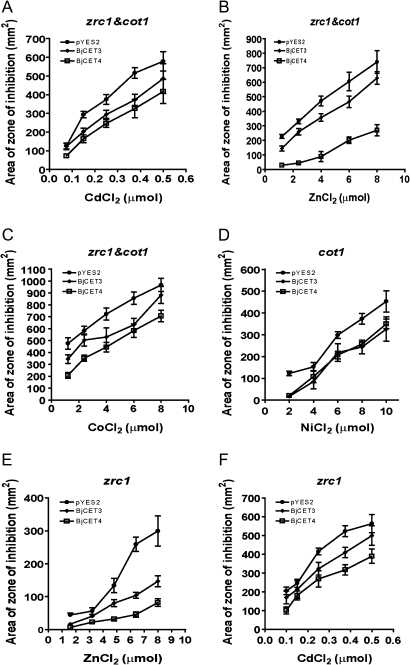
Supplementary Fig. S3 at JXB online shows representative zinc-tolerance assay plates, with zones of yeast growth inhibition being clearly visible and significantly different around the zinc ion-soaked discs between the vector control and BjCET4 expressed cot1&zrc1 (YK44) strains. Expression of BjCET3 and BjCET4 in cot1&zrc1 double mutant yeast complemented the Zn, Cd, and Co sensitivity of these strains (Fig. 4A–C), but not Ni sensitivity (data not shown). However, when expressed in the cot1 yeast strain, Ni sensitivity was rescued by the two BjCETs (Fig. 4D). The two BjCETs also complemented zrc1 yeast's sensitivity to Cd and Zn (Fig. 4E, F). In all of the yeast strains tested, the two BjCET proteins show more pronounced tolerance to Zn (Fig. 4B, E) than to Cd, Co, and Ni, and BjCET4 showed consistently higher tolerance to these metals than BjCET3. The same experiments were done with medium containing 2% glucose. No complement to the metal sensitivity of mutant yeast was observed for all the recombinant yeast strains (data not shown). In other experiments, it was also demonstrated that expression of BjCET4 and BjCET3 could restore the growth of vector control cot1 (YK40) yeast when cultured on medium containing different concentration of Zn, Ni, Co, and Cd (see Supplementary Fig. S4 at JXB online). Taken together, the results suggest that the two BjCET transporters could play a similar role to COT1 and ZRC1 in yeast, and they may either be targeted to the vacuolar membrane to sequester metal ions or targeted to the plasma membrane to export metal ions for rescuing the mutant yeast.
Fig. 4.
Quantification of metal tolerance of recombinant yeast. (A–C) Quantification of Cd, Zn and Co tolerance of zrc1&cot1 mutant strains (YK44) expressing BjCET3 and BjCET4, respectively. (D) Quantification of Ni tolerance of cot1 mutant strains (YK40) expressing BjCET3 and BjCET4. (E, F) Quantification of Zn, Cd tolerance of zrc1 mutant strains (YK41) expressing BjCET3 and BjCET4. Metal tolerance was quantified by using the assay system described in Persans et al. (2001). Data are means ±SD of three biological repeats.
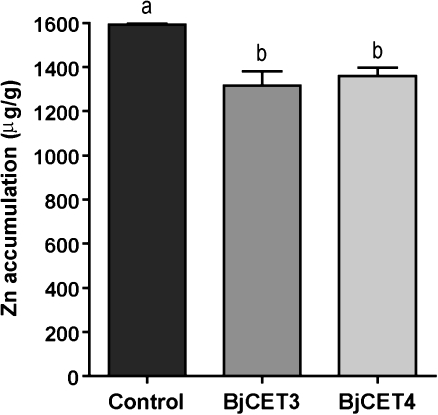
To determine the role of metal ion transport activity of BjCET3 and BjCET4, the ICP-MS method was used specifically to test for Zn accumulation in yeast cells. Yeast samples were treated with 125 μM ZnCl2 for 4 h, and the cells collected and washed with EDTA to remove zinc ions that had adhered to the cell walls. Figure 5 showed that yeast cells expressing BjCET3 (P <0.001) and BjCET4 (P <0.01) significantly reduced Zn accumulation more than the vector controls under Zn stress conditions (Fig. 5). The result suggested a plasma membrane localization of BjCET3 and BjCET4 in yeast where they could export zinc ions out of cells.
Fig. 5.
ICP-MS assay of Zn content in zrc1 yeast cells. Cultured yeast cells (OD600=0.4) were incubated with 125 μM ZnCl2 for 4 h before collection. Then yeast cells were washed with 20 mM EDTA and sterilized deionized water to remove the medium Zn and yeast cell wall unspecific bound Zn. The Zn content inside yeast cells was determined by ICP-MS. A significantly reduced Zn accumulation was detected in BjCET3 (P <0.001) and BjCET4 (P <0.01) expressing yeast compared to control (empty vector transformed) yeast. Comparison between different genotypes was made using a one-way ANOVA test to our endpoints data, followed by a Tukey test when data were statistically different (P <0.05) (Zar, 1996). Values are average of each genotype (arithmetic mean ±SD of n=3 independent experiments).
Subcellular localization of the BjCET3 and BjCET4 proteins in transgenic tobacco
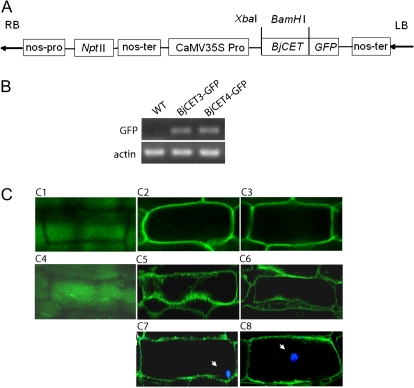
The pBI121-BjCET-GFP expression construct is designed for the constitutive expression of the BjCET genes by the enhancer-equipped CaMV 35S promoter, which is active in most cells of the plant. The kanamycin resistance gene NptII was used to screen the transformants (Fig. 6A). Using transgenic tobacco genome DNA (data not shown) and cDNA as template, PCR amplification of GFP DNA fragments confirmed that 35S–BjCET:GFP cDNA constructs were integrated into the genome of tobacco and constitutively transcribed (Fig. 6B). Using confocal microscopy, BjCET3 and BjCET4 expressed in the root of T0 transgenic tobacco seedlings was detected and their proteins had localized to the plasma membrane (Fig. 6C). To test that the BjCET:GFP proteins were not located at the cell wall, the root tissues were treated with saturated sucrose solution in order to make cell membrane plasmolyse before analysis. BjCET:GFP signals were detected which withdrew from the cell wall after 5–10 min of treatment (Fig. 6, C5 and C6). As plant root cells are usually highly vacuolated, to exclude the vacuole localization of the tested BjCET proteins, DAPI was used to stain the nucleus. Because nuclei are outside the vacuole but within the plasma membrane, if GFP-tagged proteins were localized to the vacuole, the DAPI signal should be outside the GFP signals. It was observed that the DAPI signals were surrounded by GFP signals (Fig. 6, C7 and C8) and, therefore, the BjCET:GFP proteins should not target to vacuoles. Taken together, our results indicate that BjCET3–GFP and BjCET4–GFP were located to the plasma membrane.
Fig. 6.
Verifying BjCET3 and BjCET4 expression in transgenic tobaccos and their subcellular localization in roots. (A) Structures of plasmid pBI121-BjCET-GFP. nos-pro, Nopaline synthase promoter; Npt II, neomycin phosphotransferase; nos-ter, nopaline synthase terminator; CaMV 35S Pro, CaMV 35S promoter. (B) RT-PCR analysis of BjCET3 and BjCET4 transcript level in the leaves of transgenic plants. PCR products are their fused GFP cDNA fragments. Actin was used as the control. (C) Subcellular localization of BjCET proteins in root cells of transgenic tobaccos. C1–C3, images of GFP fluorescence in transgenic tobacco root cells in normal growing condition; C4–C6, images of GFP fluorescence in transgenic tobacco root cells treated with saturated sucrose solution for 5–10 min; C7, C8, images of GFP and DAPI (arrow) fluorescence in BjCET3 and BjCET4 transgenic tobacco root cells. Before analysis, root samples were treated with 2 μg ml−1 DAPI for 15 min. C1 and C4 represent pBI121-GFP transgenic tobacco tissues, C2, C5, and C7 represent pBI121-BjCET3-GFP transgenic tobacco tissues, and C3, C6, and C8 represent pBI121-BjCET4-GFP transgenic tobacco tissues. Fluorescence of GFP and DAPI was observed by using confocal laser scanning microscopy under 485 nm and 364 nm, respectively.
BjCET3 and BjCET4 over-expression increased Cd tolerance in transgeneic tobacco
B. juncea is a Cd accumulator (Salt et al., 1995). Our yeast complementation results have shown that BjCET3 and BjCET4 expression enhanced Cd tolerance of a mutant yeast strain, which suggests the two proteins may have a role in Cd resistance and accumulation in B. juncea. As Cd is one of the most toxic heavy metals and a significant heavy metal pollutant (Oberlunder and Roth, 1978; Staessen et al., 1999), genetically engineering Cd-tolerant and -accumulating plants would have an application in phytoremediation.
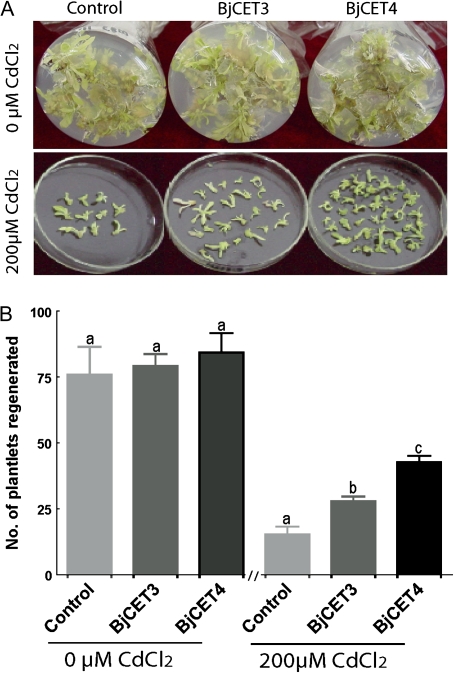
The effect of over-expressing BjCET3 and BjCET4 on the ability of tobacco leaf explants to differentiate and regenerate under Cd exposure conditions was investigated. Under normal conditions, BjCET3 and BjCET4 over-expression had no significant effect on the number of regenerated shoots among BjCET transgenic plants and vector control subjects (Fig. 7B), however, the explants had significant differences in shoot regeneration when exposed to Cd (Fig. 7A, B). BjCET4 explants regenerated the highest number of shoots, which was even more significant than BjCET3 explants (P <0.001). These results showed that over-expression of BjCET3 and BjCET4 could significantly enhance the Cd tolerance of explants, and more so with BjCET4 than with BjCET3.
Fig. 7.
Overexpression of BjCET protein confers Cd tolerance to tobacco leaf explants. (A) Showing an example of plantlets regenerated from 10 leaf explants of each genotype cultured in medium with or without 200 μM CdCl2. (B) Quantitative analysis of numbers of regenerated seedlings from each genotype. Comparison between different genotypes upon Cd exposures was made using a one-way ANOVA test to our endpoints data, followed by a Tukey test when data were statistically different (P <0.05) (Zar, 1996). Values are average number of each genotype (arithmetic mean ±SD of n=3 independent experiments). Different letters indicate significant differences between the groups. Control, pBI121-GFP vector transgenic tobacco; BjCET3, pBI121-BjCET3-GFP transgenic tobacco; BjCET4, pBI121-BjCET4-GFP transgenic tobacco. (This figure is available in colour at JXB online.)
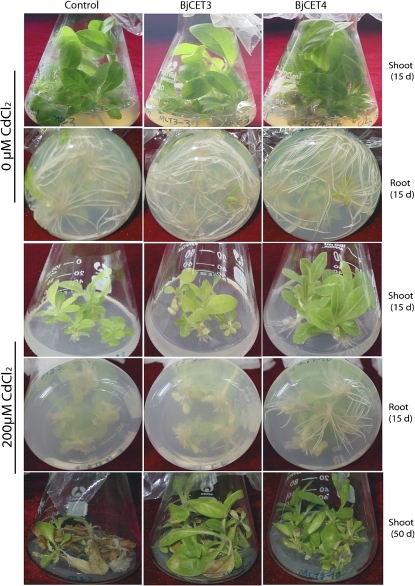
Previous studies have shown that Cd stress may significantly inhibit root growth and biomass production of plant (Balsberg Pahlsson, 1989). In our tests, under normal conditions, all the BjCET and vector control shoots grew normally and produced many roots 15 d after planting. However, when exposed to 200 μM CdCl2, no roots were observed for the vector control subjects at that stage, whereas growing roots were observed in the BjCET transgenic plants, and the BjCET4 plants grew longer roots than the BjCET3 plants (Fig. 8), suggesting that over-expression of BjCET4 makes plants more tolerant to Cd exposure than the others. After 50 d of Cd exposure, BjCET3 and BjCET4 plants survived better than the controls (Fig. 8). Under normal growing conditions, significant differences were not observed among the BjCET plants and vector control subjects at that stage (see Supplementary Fig. S5 at JXB online).
Fig. 8.
Over-expression of BiCET3 and BjCET4 enhanced Cd tolerance of transgenic tobaccos. The photographs are representative of the performance of BiCET3 and BjCET4 transgenic tobaccos on days 15 and 50 after planting on MT1 medium with or without the addition of 200 μM CdCl2. Control, pBI121-GFP vector transgenic tobacco; BjCET3, pBI121-BjCET3-GFP transgenic tobacco; BjCET4, pBI121-BjCET4-GFP transgenic tobacco. (This figure is available in colour at JXB online.)
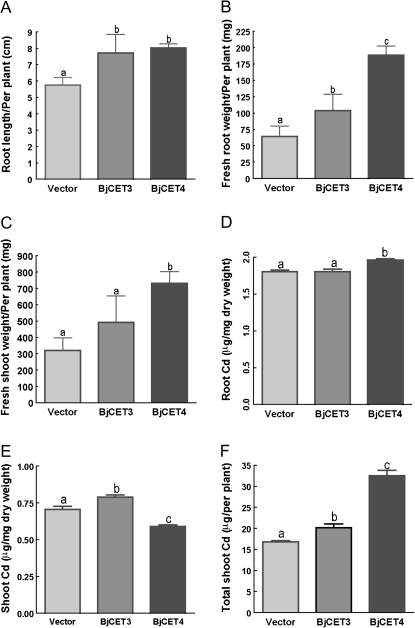
Figure 9 shows the growth index and Cd content of transgeic plants after 30 d of Cd exposure. BjCET plants have significantly longer root and higher root fresh weight in comparison with the vector control subjects (P <0.001 for BjCET4 and P <0.01 for BjCET3), and BjCET4 plants even showed a significantly higher root fresh weight than BjCET3 plants (P <0.001) (Fig. 9A, B). The shoot fresh weight of BjCET4 plants is also significantly higher than vector controls (P <0.001) and BjCET3 plants (P <0.001), while that of BjCET3 plants showed no significant difference from the vector controls (Fig. 9C).
Fig. 9.
Cd tolerance and accumulation in roots and shoots of BjCET transgenic plants exposed to CdCl2. (A–C) Quantitative analysis of root length (A), fresh root weight (B), and fresh shoot weight (C) of different genotype plantlets exposed to 200 μM CdCl2 for 30 d. (D, E) Root and shoot Cd content (μg mg−1 dry weight) of different genotype plantlets exposed to 200 μM CdCl2 for 30 d, respectively. (F) Total Cd accumulated in shoot of per plant for each genotype. The statistical analysis method used was the same as Fig. 7. Values are average number of each genotype (arithmetic mean ±SD of n=5–7). Different letters indicate significant differences between the groups.
In the analysis of the root Cd concentation (Fig. 9D), it was found that there was no difference between BjCET3 plants and vector controls, while BjCET4 plants showed about 160 μg Cd g−1 more than the vector controls (P <0.05). For the shoot Cd accumulation (Fig. 9E), BjCET3 plants showed, on average, about 90 μg Cd g−1 (dry weight) higher than the vector controls (P <0.05), while BjCET4 plants showed, on average, about 90 μg Cd g−1 (dry weight) lower than the vector controls (P <0.05). This may be a result of the higher tolerance to Cd by over-expressing BjCET4 proteins, making the shoot grow faster than others thus diluting the shoot accumulated Cd, or hte BjCET4 proteins reduced Cd accumulation in the shoot thus reducing the damage caused by the Cd poison which led to a higher shoot biomass production. The shoot/root ratios for Cd content of BjCET4, BjCET3 transgenic plants, and control subjects were 0.30±0.01, 0.43±0.02, and 0.38±0.02, respectively, and these values were significantly different from each other (P <0.05).
To compare total Cd accumulation in shoots, all the BjCET plants showed a significantly higher Cd extracting ability than the vector controls (P <0.01 for BjCET3, P <0.001 for BjCET4), and BjCET4 plants nearly accumulated 2-fold more Cd compared with the vector controls (Fig. 9F). These results showed that biomass is an important factor for phytoextracting pollutants from the soil efficiently.
Discussion
B. juncea is characterized by rapid growth, high biomass, and an appreciable capacity to take up multi-toxic metals including Cd (Rugh, 2004). The relatedness of B. juncea to numerous hyperaccumulators in the Brassicaceae family, and its ability to be self fertilized makes B. juncea a potential species for bioengineering of a practical hyperaccumulator for phytoremediation. This remains to be realized until more is known about the genes and mechanisms involved in metal uptake, detoxification, and deposition in plants. So far, five cation-efflux family members (BjCET1–4 and BjMTP1) have been identified and cloned from B. juncea, however, few have been well characterized. Sequence and phylogenetic tree analysis showed that BjCET3 and BjCET4 are more closely related than the other three cation-efflux members from B. juncea, suggesting that the two genes may have similar functions in the plant. All the five cation-efflux transporters are in the Zn-CDF group of the plant cation-efflux family, exhibit the common structural features, including six predicted transmembrane domains (TMs) and two cytoplasmic loop regions (Gaither and Eide, 2001), in which the first region is localized between TM IV and V, and include a histidine rich motif (HXn=3–6), and the second region includes the more tightly conserved motif H-D/E-X-HX-W-X-L-T-X8-H (Fig. 1A). These structural similarities suggest the five cation-efflux transporters from B. juncea, including BjCET3 and BjCET4, may have a role in the homeostasis of Zn as do the other characterized CDF family members, for example, AtMTP1 and TgMTP1.
Over-expression of BjCET3 and BjCET4 in yeast mutants deficient in their evolutionary orthologues provided clear evidence that expression of BjCET3 and BjCET4 conferred Zn resistance in the Zn-sensitive yeast mutant strains zrc1&cot1 or zrc1 (Fig. 4B, F). To approve the Zn transport activity of BjCET proteins and their roles in rescuing the metal sensitivity of mutant yeast, the ICP-MS method was used directly to test the Zn content in yeast cell cultures over-supplied with Zn. The significantly reduced Zn accumulation in BjCET-expressing yeast cells (Fig. 5) suggested the plasma membrane targeting model of BjCET proteins for exporting metal ions out of yeast cells. In addition to rescuing the Zn-sensitivity of the zrc1&cot1 and zrc1 strains, expression of BjCET3 and BjCET4 also rescued or partially rescued the Cd, Co, and Ni- sensitivity of zrc1, cot1, and/or zrc1 strains (Fig. 4A, F, C, D). Previous studies have characterized several plant Group III cation-efflux transporters in zrc1 cot1 double or single mutant yeast strains. Most plant Group III cation-efflux transporters, for example, poplar PtdMTP1 (Blaudez et al., 2003), Arabidopsis AtMTP1 (Bloss et al., 2002; Desbrosses-Fonrouge et al., 2005), AtMTP3 and AhMTP1-3 (Dräger et al., 2004; Arrivault et al., 2006), are likely to be Zn transporters capable of complementing the hypersensitivity of mutant strains to Zn but not to other metals, including Cd, Co, Mn, and Ni. Blaudez et al. (2003) identified a Leu zipper (LZ) motif in the C-terminal of PtdMTP1 sequence, which is highly conserved among plant CDFs. They found that the fourth and last Leu residue (Leu-314) of the LZ motif, together with the CDF signature sequence, are particularly critical for the function of PtdMTP1 for Zn detoxification (Blaudez et al., 2003). Both BjCET3 and BjCET4 were found to contain the conserved LZ motif and CDF sequences (Fig. 1A). To our knowledge, only T. goesingense MTP1 protein has been reported to have a much broader substrate range than AtMTP1, AhMTP1-3, and AtMTP3, which were verified to mediate tolerance to Ni, Cd, Co, and Zn in yeast cells (Persans et al., 2001). Although showing some differences in their ability to confer metal tolerance, BjCET3 and BjCET4 performed like TgMTP1- mediated tolerance to a range of metals in yeast cells. This may partially explain why B. juncea has the capability to tolerate and accumulate a range of toxic concentration of heavy metals including Zn, Cd, Co, Ni, while growing normally (Salt et al., 1995, 1997).
In B. juncea, the mRNA level of BjCET3 and BjCET4 were found to be transcriptionally regulated not only by heavy metal stresses like Cd2+ and Zn2+, but also by osmotic stresses like salinity (NaCl) and PEG, a different expression pattern compared with currently characterized Group III cation-efflux transporters, for example, AtMTP1 from non-accumulator A. thaliana (Desbrosses-Fonrouge et al., 2005), TgMTP1 from hyperaccumulator T. goesingense (Persans et al., 2001; Kim et al., 2004; Gustin et al., 2009), and BjCET2 (Xu et al., 2009) and BjMTP1 (Muthukumar et al, 2007) from B. juncea. PEG is an inert, non-ionic, and non-penetrating compound of high molecular weight, which is widely used to simulate water-deficit conditions in a similar manner to that observed in the cells of intact plants subjected to true drought conditions (Hohl and Schopfer, 1991; Attree et al., 1991). In B. juncea, BjMTP1 is strongly regulated by Cd2+ and Ni2+, and weakly regulated by Zn2+, but not the other forms of abiotic stress including cold, heat, and NaCl (Muthukumar et al., 2007). Our previous work also demonstrated that the BjCET2 mRNA level is regulated by Cd and Zn, but not cold, salt, and drought (Xu et al., 2009), which showed that BjCET2 and BjMTP1 have similar functions, however, no available in vivo functional data for BjMTP1 have been reported at present. As B. juncea is an amphidiploid plant resulting from the hybridization of B. nigra and B. campestris (syn. rapa), which contains the conserved genomes from both of its diploid parents (Muthukumar et al., 2007), and our phylogenetic tree analysis also showed that BjCET2 and BjMTP1 are the most closely related (Fig. 1B), this suggested that the two genes may be the result of a relatively recent gene amplification or that the orthologues from the parent species. BjCET3 and BjCET4 may be a similar case due to their similar functions and relationship (Fig. 1B).
In A. thaliana, to date all studies have found that AtMTP1 expression is not modulated by exposure to elevated Zn2+, Cd2+, Co2+, Cu2+, Fe2+ or Mn2+ (van der Zaal et al., 1999; Desbrosses-Fonrouge et al., 2005), while AtMTP3 expression is up-regulated under Fe deficiency, and under conditions of excess Zn2+ or Co2+, which showed some similarity with BjCET3 and BjCET4. In poplar, PtdMTP1 mRNA was expressed constitutively throughout hybrid poplar and was not regulated by low or high concentrations of Zn, and also no response to Cd, Ni, and drought stress were found (Blaudez et al., 2003). In hyperaccumulators, AhMTP1 transcript levels did not show changes following exposure to Co, Cd, Cu or H2O2 in either the roots or shoots of A. halleri, but was up-regulated specifically in response to high Zn concentrations in the root (Dräger et al., 2004). Expression of the Ni transporter TgMTP1 from T. goesingense failed to increase during Ni exposure, although it is a MTP1-related Ni hyperaccumulator (Persans et al., 2001). Similarly, BjCET3 and BjCET4, like their homologues AtMTP1, AtMTP3, and PtdMTP1 in non-accumulators, showed low transcript levels under normal conditions. Comparatively, BjCET3 is more expressed than BjCET4 in the root, stem, and leaf of B. juncea, and both BjCET3 and BjCET4 accumulate relatively more in the root than the other tissues, which is similar to AtMTP3 transcript abundance (Arrivault et al., 2006). MTP1 is much more highly expressed in the hyperaccumulators (e.g. A. halleri, T. caerulescens, and T. goesingense) than in non-accumulator species (e.g. A. thaliana, poplar), presumably conferring enhanced ability to accumulate high concentration of metal ions, which exhibits a prominent character in the hyperaccumulator species (Kim et al., 2004).
Through imaging BjCET–GFP fusions in transgenic tobacco root cells, BjCET3 and BjCET4 were localized to the plasma membrane, and our ICP-MS data also suggested the plasma membrane targeting model of the two BjCETs in yeast. To verify that the BjCET:GFP fused protein does not lose the metal transport function, the BjCET GFP fused protein was expressed in mutant yeast under Zn stress conditions, and similar rescue results were obtained as the non-GFP fused BjCET proteins (see Supplementary Fig. S6 at JXB online; Fig. 4E). Furthermore, it was shown that constitutive over-expression of BjCET3 and BjCET4 markedly enhanced the Cd tolerant ability of transgenic tobaccos. However, the two transporters may have different mechanisms to acquire the Cd-tolerant ability, compared with control subjects. BjCET3 over-expressing plants accumulated significant amounts of Cd in shoots while producing similar amounts of biomass. However, BjCET4 over-expressing plants markedly increased shoot biomass production with significantly reduced Cd content in shoot. The previous finding of the Zn-induced membrane relocalization of CDF protein ZNT6 from the trans Golgi network (TGN) to the plasma membrane may aid in this interpretation (Huang et al., 2002). Several other reports also showed a variable location of CDF transporters. For example, the S. hamata CDF transporter ShMTP1 was found to be targeted to the vacuolar membrane in planta but localized to the endoplasmic reticulum membranes when expressed in yeast (Delhaize et al., 2003), TgMTP1 from T. goesingense was localized to both the plasma membrane (in yeast cells and protoplasts of A. thaliana shoot tissues) and vacuolar membranes (in A. thaliana and T. goesingense shoot tissues) (Kim et al., 2004; Gustin et al., 2009). The LZ motifs presented in ZNT6 are suggested to be involved in membrane relocalization and because LZ motifs are very conserved in Group III cation-efflux members and critical to the function of Zn efflux (Blaudez et al., 2003), the dynamic relocalization of CDF proteins between the plasma membrane and various endomembrane systems may play a critical role in Zn and/or other metal ion homeostasis and hyperaccumulation in metal hyperaccumulators (Huang et al., 2002; Kim et al., 2004).
In conclusion, our study of the B. juncea BjCET3 and BjCET4 genes strongly indicates that they are functional and evolutionary orthologues with similar protein functions. In addition to Zn, the two proteins may also be involved in regulating Cd, Co or Ni metal ions homeostasis in B. juncea, which function in a manner similar to TgMTP1 from T. goesingense. Their strong up-regulation in response to both Zn and Cd of heavy metal stresses and NaCl and PEG of osmotic stresses, suggests a novel role in balancing the ionic aspects of osmotic stress and transition metal homeostasis, which may not occur for other characterized cation-efflux members, although this role is not clear at present. Both BjCET3 and BjCET4 proteins were localized to the plasma membrane in root cells of transgenic tobaccos, and over-expression of BjCET3 and BjCET4 markedly enhanced the Cd-tolerant ability of transgenic plants, and greatly changed the Cd accumulation process in shoots. BjCET4 over-expressing plants showed a reduced shoot Cd content under Cd excess conditions, however, because of its significantly enhanced shoot biomass production, the single BjCET4 plant still accumulated much more Cd in the shoot than the others. Therefore, biomass is an important factor for phytoextracting pollutants from the soil efficiently. The two factors of metal concentration and shoot biomass should be balanced in order to achieve a high efficiency of phytoremediation.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Multiple alignment analysis of deduced amino acid sequences of the CE proteins.
Supplementary Fig. S2. Testing the effect of Cd stress on the growth of B. juncea.
Supplementary Fig. S3. A representative of the zinc-tolerance assay plates.
Supplementary Fig. S4. Analysis of the effect of expressing BjCET3 and BjCET4 in cot1 mutant yeast with over-supplied metal ions.
Supplementary Fig. S5. The photographs show representative performance of BiCET3, BjCET4, and empty vector (control) transgenic tobaccos at day 50 of growing in normal conditions.
Supplementary Fig. S6. Testing the exporting activity of BjCET:GFP proteins.
Supplementary Table S1. Topology and hydrophile analysis of putative proteins of CE members from B. juncea and other species.
Supplementary Table S2. Gene name, GenBank accession number and species.
Acknowledgments
This work was supported by grants from the National Program of Research and Development of the Transgenic Plants of China (No. 2009ZX08002-012B, JY03A2002), and the China Postdoctoral Science Foundation (No. 20060390479). We thank the United States National Plant Germplasm System for providing seeds of B. juncea L and Professors Bing Zhou (Tsinghua University), Tuanyao Chai (Graduate University, CAS), Michael Kanost (Kansas State University), and Wenjun Ding and Wei Wang (Graduate University, CAS) for providing material support for this study. We thank Professor Gerald Reeck (Kansas State University) for his comments on the manuscript.
References
- Arrivault S, Senger T, Krämer U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. The Plant Journal. 2006;46:861–879. doi: 10.1111/j.1365-313X.2006.02746.x. [DOI] [PubMed] [Google Scholar]
- Assucao AG, Costa Martins PDA, Folter SDE, Vooijs R, Schat H, Aarts MGM. Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant, Cell and Environment. 2001;24:217–226. [Google Scholar]
- Attree SM, Moore D, Sawhney VK, Fowke LC. Enhanced maturation and desiccation tolerance of white spruce (Picea glauca (Moench) Voss) somatic embryos: effect of a non-plasmolysing water stress and abscisic acid. Annals of Botany. 1991;68:519–522. [Google Scholar]
- Balsberg Pahlsson A-M. Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants. Water, Air, and Soil Pollution. 1989;47:287–319. [Google Scholar]
- Blaudez D, Kohler A, Martin F, Sanders D, Chalot M. Poplar metal tolerance protein 1 confers zinc tolerance and is an oligomeric vacuolar zinc transporter with an essential leucine zipper motif. The Plant Cell. 2003;15:2911–2928. doi: 10.1105/tpc.017541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss T, Clemens S, Nies DH. Characterization of the ZAT1 p zinc transporter from Arabidopsis thaliana in microbial model organisms and reconstituted proteoliposomes. Planta. 2002;214:783–791. doi: 10.1007/s00425-001-0677-1. [DOI] [PubMed] [Google Scholar]
- Chaney RL. Plant uptake of inorganic waste. In: Parr JE, Marsh PB, Kla JM, editors. Land treatment of hazardous wastes. Park Ridge, Illinois, USA: Noyes Data; 1983. pp. 50–76. [Google Scholar]
- Curie C, Alonson JM, Le JM. Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochemistry Journal. 2000;347:749–755. [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Kataoka T, Hebb DM, White RG, Ryan PR. Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. The Plant Cell. 2003;15:1131–1142. doi: 10.1105/tpc.009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Pittman JK, White RG, Leung H, Miao Y, Jiang L, Ryan PR, Richardson AE. A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. The Plant Journal. 2007;51:198–210. doi: 10.1111/j.1365-313X.2007.03138.x. [DOI] [PubMed] [Google Scholar]
- Desbrosses-Fonrouge AG, Voigt K, Schröder A, Arrivault S, Thomine S, Krämer U. Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS Letters. 2005;579:4165–4174. doi: 10.1016/j.febslet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Dietz K-J, Baier M, Kramer U. Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In: Prasad MNV, Hagemeyer J, editors. Heavy metal stress in plants: from molecules to ecosystems. Berlin: Springer-Verlag; 1999. pp. 73–97. [Google Scholar]
- Dräger DB, Desbrosses-Fonrouge AG, Krach C, Chardonnens AN, Meyer RC, Saumitou-Laprade P, Krämer U. Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. The Plant Journal. 2004;39:425–439. doi: 10.1111/j.1365-313X.2004.02143.x. [DOI] [PubMed] [Google Scholar]
- Filatov V, Dowdle J, Smirnoff N, Ford-Lloyd B, Newbury HJ, Macnair MR. Comparison of gene expression in segregating families identifies genes and genomic regions involved in a novel adaptation, zinc hyperaccumulation. Molecular Ecology. 2006;15:3045–3059. doi: 10.1111/j.1365-294X.2006.02981.x. [DOI] [PubMed] [Google Scholar]
- Ford D. Intestinal and placental zinc transport pathways. Proceedings of the Nutrition Society. 2004;1:21–29. doi: 10.1079/PNS2003320. [DOI] [PubMed] [Google Scholar]
- Gaither LA, Eide DJ. Eukaryotic zinc transporters and their regulation. Biometals. 2001;14:251–270. doi: 10.1023/a:1012988914300. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. High efficiency transformation in yeast. In: Johnston JA, editor. Molecular genetics of yeast: a practical approach. New York: Oxford University Press; 1994. pp. 121–134. [Google Scholar]
- Godbold DL, Hüttermann A. Effect of zinc, cadmium and mercury on root elongation of Picea abies (Karst.) seedlings, and the significance of these metals to forest dieback. Environmental Pollution. 1985;38:375–381. [Google Scholar]
- Guerino TML. The ZIP family of metal transporters. Biochimica et Biophysica Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Gustin JL, Loureiro ME, Kim D, Na G, Tikhonova M, Salt DE. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. The Plant Journal. 2009;57:1116–1127. doi: 10.1111/j.1365-313X.2008.03754.x. [DOI] [PubMed] [Google Scholar]
- Hall JL, Williams LE. Transition metal transporters in plants. Journal of Experimental Botany. 2003;54:2601–2613. doi: 10.1093/jxb/erg303. [DOI] [PubMed] [Google Scholar]
- Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ. Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiology. 2000;124:125–134. doi: 10.1104/pp.124.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl M, Schopfer P. Water relations of growing maize coleoptiles. Plant Physiology. 1991;95:716–722. doi: 10.1104/pp.95.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method of transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Huang L, Kirschke CP, Gitschier J. Functional characterization of a novel mammalian zinc transporter, ZnT6. Journal of Biological Chemistry. 2002;277:26389–26395. doi: 10.1074/jbc.M200462200. [DOI] [PubMed] [Google Scholar]
- Kim D, Gustin JL, Lahner B, Persans MW, Baek D, Yun DJ, Salt DE. The plant CDF family member TgMTP1 from the Ni/Zn hyperaccumulator Thlaspi goesingense acts to enhance efflux of Zn at the plasma membrane when expressed in Saccharomyces cerevisiae. The Plant Journal. 2004;39:237–251. doi: 10.1111/j.1365-313X.2004.02126.x. [DOI] [PubMed] [Google Scholar]
- Kim YY, Choi H, Segami S, Cho HT, Martinoia E, Maeshima M, Lee Y. AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. The Plant Journal. 2009;58:737–753. doi: 10.1111/j.1365-313X.2009.03818.x. [DOI] [PubMed] [Google Scholar]
- Kobae Y, Uemura T, Sato MH, Ohnishi M, Mimura T, Nakagawa T, Maeshima M. Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuolar membranes and implicated in zinc homeostasis. Plant and Cell Physiology. 2004;45:1749–1758. doi: 10.1093/pcp/pci015. [DOI] [PubMed] [Google Scholar]
- Krämer U, Talke IN, Hanikenne M. Transition metal transport. FEBS Letters. 2007;581:2263–2272. doi: 10.1016/j.febslet.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Lang ML, Zhang YX, Chai TY. Identification of genes up-regulated in response to Cd exposure in Brassica juncea L. Gene. 2005b;363:151–158. doi: 10.1016/j.gene.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Lang ML, Zhang YX, Guan ZQ, Chai TY. PCR-enriched cDNA pool method for cloning of gene homologues. Plant Molecular Biology Reporter. 2005a;23:219–226. [Google Scholar]
- Milner MJ, Kochian LV. Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Annals of Botany. 2008;102:3–13. doi: 10.1093/aob/mcn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics. 2007;8:107. doi: 10.1186/1471-2164-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumar B, Yakubov B, Salt DE. Transcriptional activation and localization of expression of Brassica juncea putative metal transport protein BjMTP1. BMC Plant Biology. 2007;18:32. doi: 10.1186/1471-2229-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies DH. Microbial heavy-metal resistance. Applied Microbiology and Biotechnology. 1999;51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- Nies DH, Silver S. Ion efflux systems involved in bacterial metal resistances. Journal of Industrial Microbiology and Biotechnology. 1995;14:186–199. doi: 10.1007/BF01569902. [DOI] [PubMed] [Google Scholar]
- Oberlunder HE, Roth K. Toxic metals in soil–plant systems. New York, USA: Wiley; 1978. Toxic metals in soil–plant systems; pp. 12293–12301. [Google Scholar]
- Paulsen IT, Saier MH. A novel family of ubiquitous heavy metal ion transport proteins. Journal of Membrane Biology. 1997;156:99–103. doi: 10.1007/s002329900192. [DOI] [PubMed] [Google Scholar]
- Peiter E, Montanini B, Gobert A, Pedas P, Husted S, Maathuis FJ, Blaudez D, Chalot M, Sanders D. A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proceedings of the National Academy of Sciences, USA. 2007;104:8532–8537. doi: 10.1073/pnas.0609507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persans MW, Nieman K, Salt DE. Functional activity and role of cation-efflux family members in Ni hyperaccumulation in Thlaspi goesingense. Proceedings of the National Academy of Sciences, USA. 2001;98:9995–10000. doi: 10.1073/pnas.171039798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigola D, Fiers M, Vurro E, Aarts MGM. The heavy metal hyperaccumulator Thlaspi caerulescens expresses many species-specific genes, as identified by comparative expressed sequence tag analysis. New Phytologist. 2006;4:753–765. doi: 10.1111/j.1469-8137.2006.01714.x. [DOI] [PubMed] [Google Scholar]
- Rugh CL. Genetically engineered phytoremediation: one man's trash is another man's transgene. Trends in Biotechnology. 2004;22:496–498. doi: 10.1016/j.tibtech.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Salt DE, Pickering IJ, Prince RC, Gleba D, Dushenkov S, Smith R, Raskin I. Metal accumulation by aquacultured seedlings on Indian mustard. Environmental Science and Technology. 1997;31:1636–1644. [Google Scholar]
- Salt DE, Prince RC, Pickering IJ, Raskin I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiology. 1995;109:1427–1433. doi: 10.1104/pp.109.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Roels HA, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R. Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group. Lancet. 1999;353:1140–1144. doi: 10.1016/s0140-6736(98)09356-8. [DOI] [PubMed] [Google Scholar]
- Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proceedings of the National Academy of Sciences, USA. 2000;97:4991–4996. doi: 10.1073/pnas.97.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chai TY, Zhang YX, Lang ML, Han L. The cation-efflux transporter BjCET2 mediates zinc and cadmium accumulation in Brassica juncea L. leaves. Plant Cell Reports. 2009;28:1235–1242. doi: 10.1007/s00299-009-0723-1. [DOI] [PubMed] [Google Scholar]
- Van der zaal BJ, Neuteboom LW, Pina JE. Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiology. 1999;199:1047–1055. doi: 10.1104/pp.119.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkerpeach CR, Velten J. Plant molecular biology manual. Dordrecht, The Netherlands: Kluwer B1; 1994. Agrobacterium-mediated gene transfer to plant cells: co-integrate and binary vector systems; pp. 1–19. [Google Scholar]
- Zar JH. Biostatistical analysis. Upper Saddle River, NJ, USA: Prentice-Hall; 1996. pp. 162–184. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.