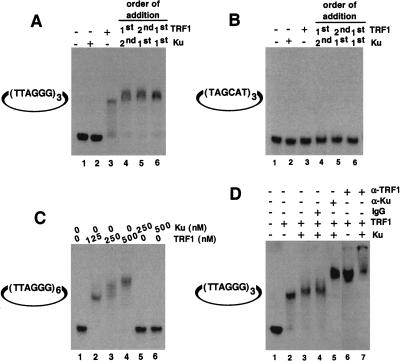
Figure 3.
Telomeric DNA binding analysis by EMSA. (A) Ku does not bind directly to internal telomeric repeats. Binding reactions were performed as described above using a mirocircle containing (TTAGGG)3 repeats. Lane 1, no protein added; lane 2, 250 nM Ku70/Ku80 only; lane 3, 125 nM TRF1 alone; lane 4, 250 nM Ku was incubated with substrate DNA first, followed by 125 nM TRF1; lane 5, 125 nM TRF1 was incubated with substrate DNA first, followed by 250 nM Ku; lane 6, a preformed complex containing 250 nM Ku and 125 nM TRF1 was incubated with substrate DNA. (B) Microcircles containing random sequence repeats were not bound by both TRF1 and Ku. Reaction mixtures are same as A except that the substrate used was a microcircle containing a random sequence (TAGCAT)3 repeat. (C) Titration of binding sites. Binding studies were performed using a microcircle containing (TTAGGG)6 repeats. Lane 1, no protein; lane 2, 125 nM TRF1; lane 3, 250 nM TRF1; lane 4, 500 nM TRF1; lane 5, 250 nM Ku; lane 6, 500 nM Ku. (D) Antibody mediated super-shift. The telomeric microcircle DNA containing (TTAGGG)3 repeats was incubated with either TRF1 alone (lane 2) or as a preformed complex of TRF1 with Ku70/Ku80 (lanes 3–5,7) as described above, except that the concentration of the poly (dI/dC) was reduced to 0.1 mg/mL. Appropriate antibody was then added to individual reaction mixtures, and the resulting DNA–protein complexes were resolved as described in methods. As a control, mouse IgG2a was added to one of the reactions (lane 4). Rabbit α-TRF1 antibody (lanes 6,7) or mouse α-Ku70/Ku80 (lane 5) antibodies were used.