Figure 4.
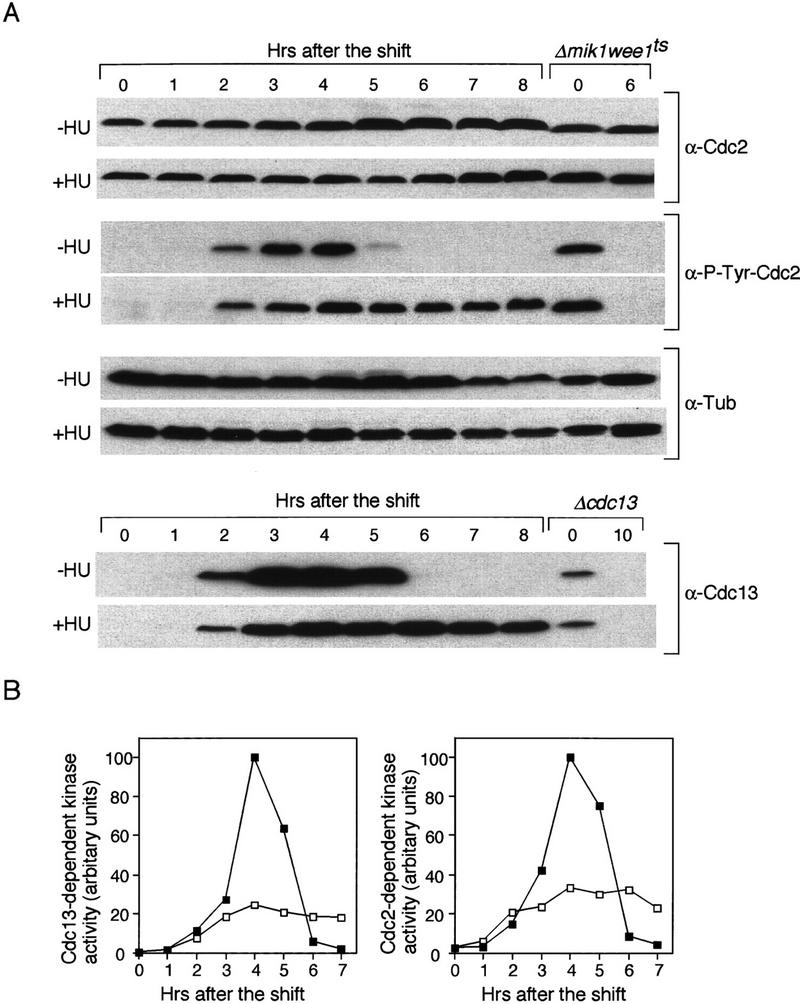
The meiotic DNA replication checkpoint inhibits Cdc2 kinase activity through Y15 phosphorylation. (A) Western blot analysis of the amount of tyrosine 15 phosphorylated Cdc2, and the abundance of Cdc2 and Cdc13 proteins. pat1ts/pat1ts cells were induced to undergo meiosis as in Fig. 2. The samples were taken after the temperature shift at the indicated times in the absence (−HU) or in the presence (+HU) of HU. The levels of tyrosine 15 phosphorylated Cdc2 in the samples were monitored by Western blotting with an antibody specific to tyrosine 15 phosphorylated Cdc2 (α-P-Tyr-Cdc2). As a control for Y15 phosphorylated Cdc2, Δmik1 weelts strain was grown to mid log phase, shifted to 36°C at time 0 and incubated for 6 hr. The blots were reprobed with a monoclonal antibody specific to Cdc2 (α-Cdc2). As a loading control, the amount of α-tubulin was analyzed α-Tub). Similarly, the amount of Cdc13 was analyzed (α-Cdc13). As a control for Cdc13 protein, Δcdc13 strain in which cdc13+ expression is repressible by thiamine was grown in mid log phase. Thiamine was added to shut off the expression of cdc13+ at time 0 and incubated for 10 hr. (B) pat1ts/pat1ts cells were grown and processed as above. Timing of entry into meiosis was delayed for ∼0.5 hr compared with that in A. Kinase activity associated with Cdc13 and Cdc2 was analyzed by immunoprecipitation with anti-Cdc13 and anti-Cdc2 antibodies from the samples in the absence (█) or presence (□) of HU. Immune complexes were washed, assayed for kinase activity with histone H1 as a substrate, and quantified by PhosphorImager analysis.
