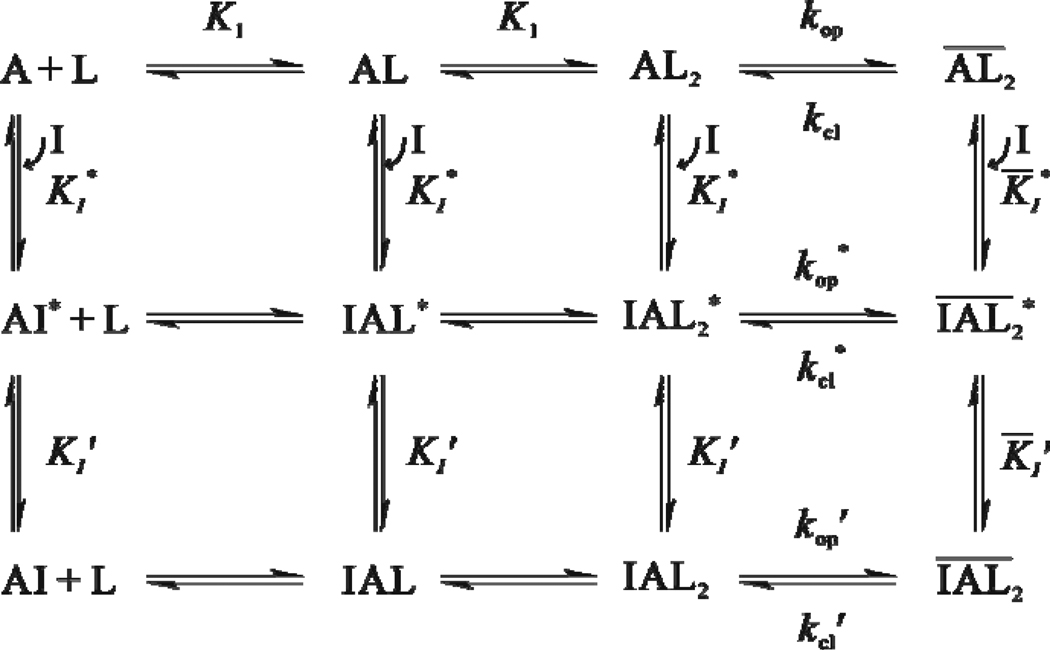
Figure 4.
A minimal mechanism of inhibition for BDZ-f. The upper row shows the channel-opening reaction of the AMPA receptor. A represents the active, unliganded form of the receptor, L the ligand (glutamate), AL and AL2 the ligand-bound closed-channel forms, the open-channel form or the state of the receptor (all the species with a bar sign refer to open-channel state), kop the channel-opening rate constant, and kcl the channel-closing rate constant. For simplicity and without contrary evidence, it is assumed that glutamate binds to the two steps with equal affinity, represented by the same intrinsic equilibrium dissociation constant, K1. The initial binding of BDZ-f to the receptor is assumed to form a loosely bound, partially conducting intermediate (e.g., ) in both the closed-channel and open-channel states of the receptor (middle row). In the second step (from the middle to the lower row), the receptor:inhibitor intermediate rapidly isomerizes into a more tightly bound complex , and such a complex is no longer capable of conducting ions. The inhibition constants pertinent to various steps in this mechanistic scheme are shown in Table 1. KI represents the overall inhibition constant associated with the closed-channel state of the receptor (i.e., the values from Column 5 in Table 1), K̅I, the overall inhibition constant associated with open-channel state (i.e., the values from Column 6 in Table 1), I, the inhibitor, kop' the channel-opening rate constant of the inhibited AMPA receptor, kcl' the channel-closing rate constant of the inhibited AMPA receptor. In addition, the values for and for step 1 can be found from Columns 1 and 2 in Table 1.