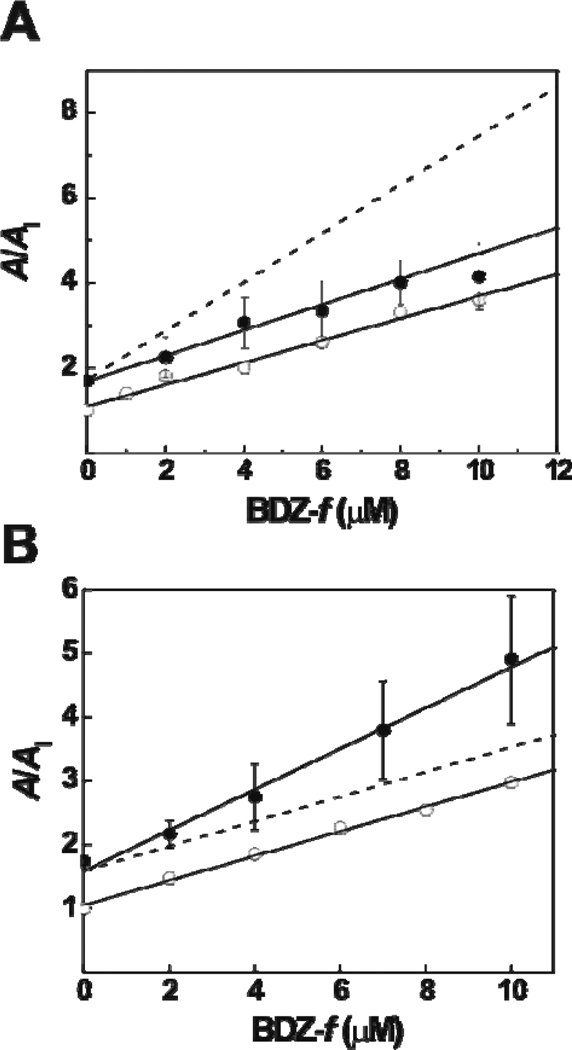
Figure 6.
(A) The result of a double-inhibition experiment for GYKI 52466 and BDZ-f on GluA2Qflip determined at 100 µM-glutamate concentration. The concentration of GYKI 52466 was fixed at 15 µM. The double-inhibition constant, KI′, was determined to be 3.6 ± 0.6 µM (filled circles), compared with KI of 3.8 ± 0.4 µM for BDZ-f alone (open circles). The dashed line simulates the A/AI ratio by using from eq 8, assuming that the two inhibitors bind to two different sites with a double-inhibition constant of ~1.9 µM (when GYKI 52466 was fixed at 15 µM). (B) The result of the double-inhibition experiment for BDZ-2 and BDZ-f on GluA2Qflip determined at 3 mM-glutamate concentration. The double-inhibition constant, K̅I′, was determined to be 3.1 ± 0.2 µM (filled circles) compared with K̅I of 5.4 ± 0.8 µM for BDZ-f alone (open circles). The dashed line represents the simulated A/AI values by using eq 7, assuming that the two inhibitors bind to the same site with an inhibition constant of ~5.4 µM (when BDZ-2 was fixed at 8 µM).