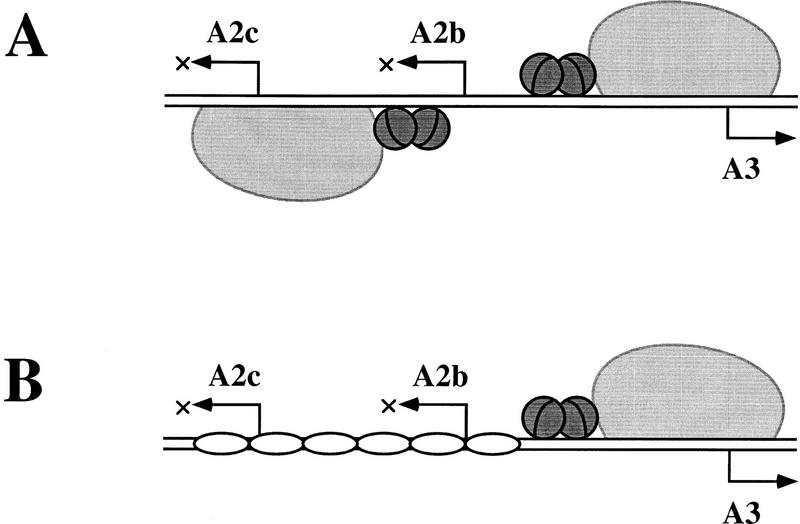
Figure 8.
Two different strategies for regulating the early–late transcriptional switch of phage φ29 in vitro. (A) In the absence of protein p6, repression of the early A2b and A2c promoters and activation of the late A3 promoter is directly mediated by protein p4 and its physical interaction with RNAP. Protein p4 activates PA3 by binding to its site at this promoter and stabilizing RNAP at PA3 as a closed complex. Binding of p4 to this site also entails repression of PA2b, whose −35 region overlaps with the p4-binding site at PA3. Repression of PA2c, on the other hand, stems from p4 binding to its site at PA2c and holding the RNAP at the promoter (for review, see Rojo et al. 1998). (B) In the presence of p6 and p4, p6 appears as the direct mediator of PA2c repression by forming a nucleoprotein complex that precludes RNAP binding to this promoter. Moreover, in the presence of p6, binding of p4 to its site at PA3 is enhanced, as is PA3 activation and PA2b repression. The cartoon shows RNAP as a large light gray protein; the small dark gray spheres depict protein p4 dimers, and the unshaded oblate ellipses represent protein p6 dimers. For simplicity, the complexed DNA is drawn as a straight rod.