Table 1.
Effect of iodinating reagents, solvents and temperature on the iodination of 3,5-dichlorophenol (1b).*
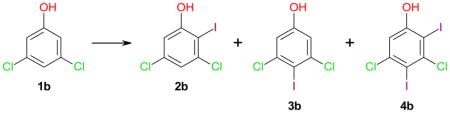 | ||||||
|---|---|---|---|---|---|---|
| Entry | Reaction Conditionsa | Reaction time (h) | Conversion (%) | Yield
|
||
| 2b (%) | 3b (%) | 4b (%) | ||||
| 1–1 | I2 (1.5 eq.), C2H5OH | 16 | 100 | 16 | 1 | T |
| 1–2 | N-Iodosuccinimide, PTSA, CH3CN | 24 | < 100 | 18 | 57 | T |
| 1–3 | BTMACl2I, ZnCl2, AcOH, RTb | 24 | < 100 | 5 | 4 | nd |
| 1–4 | BTMACl2I, ZnCl2, AcOH, 90 °Cc | 2 | < 100 | 46 | 39 | T |
| 1–5 | CAN, I2, CH3CN | 24 | > 0 | 2 | 2 | nd |
| 1–6 | Ag2SO4, I2, CH3CNd | 16 | > 0 | 11 | 3 | T |
| 1–7 | Ag2SO4, I2, DCM-MeOH-H2O (1:1:1, v/v)d | 2 | 100 | 9 | 2 | T |
| 1–8 | Ag2SO4, I2, β-cyclodextrine | 1 | 100 | 8 | nd | nd |
| 1–9 | Ag2SO4, I2, n-hexaned | 16 | < 100 | 49 | 41 | T |
| 1–10 | Ag(OCOCF3)2, I2, C2H5OH | 16 | < 100 | 28 | 4 | T |
Percent conversion and yields were determined by GC-MS;
one equivalent (eq.) of each key reagent was employed if not mentioned otherwise;
BTMACl2I (1.5 eq.) and ZnCl2 (1.5 eq.);
BTMACl2I (1.1 eq.) and ZnCl2 (1.5 eq.);
I2 (1.5 eq.) and Ag2SO4 (1.1 eq.);
β-cyclodextrin in DMSO was added to a solution containing 1b and Ag2SO4/I2 (1 eq.: 1 eq.) in DCM (DMSO: DCM = 1: 1, v/v);
T = traces were detected by GC-MS; nd = not detected; BTMACl2I = benzyltrimethylammonium dichloroiodinate; RT = room temperature; PTSA = p-toluenesulfonic acid.
