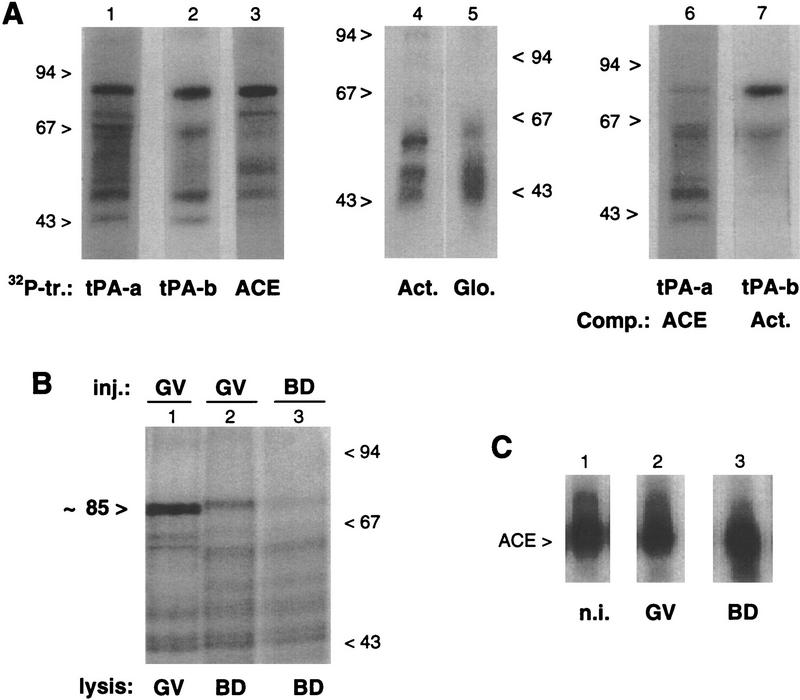
Figure 6.
In vivo UV-induced cross-linking of 32P-labeled 3′ UTR transcripts. (A) Primary oocytes were injected with different 32P-labeled transcripts (tr.) from the 3′ UTRs of tPA (tPA-a, tPA-b, and ACE, lanes 1–3), β-actin (Act., lane 4) and β-globin (Glo., lane 5) mRNAs. Alternatively, primary oocytes were coinjected with 32P-labeled tPA-a and the ACE competitor (Comp.) fragment (at a 50-fold molar excess) (lane 6), or with 32P-labeled tPA-b and the β-actin 3′ UTR fragment (at a 20-fold molar excess) (lane 7). Following 5 hr of culture in the presence of dB-cAMP, the oocytes were exposed to UV light and lysed. The samples were digested with RNase T1 and analyzed by SDS-PAGE and autoradiography. The migration of molecular-mass markers is indicated in kD. (B) In maturing oocytes, primary oocytes were injected with the 32P-labeled ACE transcript and cultured for 4 hr in medium with (lane 1) or without (lane 2) dB-cAMP, before being exposed to UV light. Maturing oocytes cultured without dB-cAMP were injected with the 32P-labeled ACE transcript 2 hr after GVBD and exposed to UV light 2 hr later (lane 3). Following lysis of the oocytes, the samples were processed and analyzed as for A. (C) ACE transcript in injected oocytes. Primary oocytes injected with the 32P-labeled ACE transcript were cultured for 6 hr in the presence (lane 2) or absence (lane 3) of dB-cAMP. The 32P-labeled transcripts recovered from arrested primary oocytes (GV, lane 2) or maturing oocytes (BD, lane 3) were analyzed on a 6% acrylamide–urea gel. In lane 1, the noninjected (n.i.) 32P-labeled ACE transcript was analyzed in parallel.