Abstract
Real-time ultrasound perfusion imaging (rt-UPI) allows visualization of microbubbles flowing through the cerebral microvasculature. We hypothesized that analysis of microbubble tissue replenishment would enable for characterization of perfusion deficits in acute middle cerebral artery (MCA) territory stroke. Twenty-three patients (mean age 70.2±13.2 years, 9 weeks) were included. Sequential images of bubble replenishment were acquired by transcranial rt-UPI at low mechanical index immediately after microbubble destruction. Different parameters were calculated from regions of interest (ROIs): real-time time to peak (rt-TTP), rise rate (β), and plateau (A) of acoustic intensity, and A × β was used as an index of blood flow. Results were compared with diffusion-weighted and perfusion magnetic resonance imaging. Parameters of rt-UPI had lower values in ROIs of ischemic as compared with normal tissue (β=0.58±0.40 versus 1.25±0.83; P=0.001; A=1.44±1.75 versus 2.63±2.31; P=0.05; A × β=1.14±2.25 versus 2.98±2.70; P=0.01). Real-time time to peak was delayed in ischemic tissue (11.43±2.67 versus 8.88±1.66 seconds; P<0.001). From the analysis of receiver operating characteristic curves, β and A × β had the largest areas under the curve with optimal cutoff values of β<0.76 and A × β<1.91. We conclude that rt-UPI with analysis of microbubble replenishment correctly identifies ischemic brain tissue in acute MCA stroke.
Keywords: acute stroke, brain imaging, cerebral hemodynamics, neurosonology, ultrasound
Introduction
Assessment of brain perfusion in acute stroke is important because it allows early estimation of infarct extension and severity; in particular, magnetic resonance imaging (MRI) perfusion imaging can be used to select patients for thrombolysis in an extended time window (Hjort et al, 2005; Kidwell et al, 2003). Other perfusion imaging techniques such as single photon emission computed tomography, positron emission tomography, and xenon-computed tomography are available for research issues, but have the disadvantage of being time consuming and technically demanding (Heiss et al, 2000; Wintermark et al, 2005; Olivot et al, 2009).
Ultrasound perfusion imaging (UPI) can likewise identify perfusion deficits in the brain parenchyma (Seidel et al, 2000; Meairs et al, 2000; Federlein et al, 2000). A significant advantage of UPI is its capability of noninvasive bedside monitoring of cerebral perfusion. Ultrasound perfusion imaging, however, has been limited to semiquantitative studies for characterization of perfusion abnormalities in stroke patients (Kern et al, 2004; Krogias et al, 2005; Eyding et al, 2006). This stands contrary to applications of UPI in other organs such as the heart (Wei et al, 1998; Elhendy and Porter, 2005), kidney (Schlosser et al, 2001; Wei et al, 2001), and skeletal muscle (Vincent et al, 2002), where excellent in vivo measurements of tissue blood flow have been obtained by implementation of microbubble refill/replenishment kinetics (Wei et al, 1998).
Refill kinetics are based on the reappearance of echo-contrast agents in tissue after complete microbubble destruction. By destroying the contrast agent within the scanning plane using a high mechanical index (MI), a negative bolus of contrast agent is created locally and new microbubbles enter the ultrasound beam with a certain velocity within a certain volume of tissue, thus allowing calculation of regional blood flow. Rim et al (2001) used microbubble replenishment kinetics to measure regional cerebral blood flow (rCBF) in dogs. Different from the situation in humans, however, the authors scanned the brain through a craniotomy in the parietal bone, thus allowing for bubble destruction with high MI followed by real-time low MI imaging of replenishment in the dog brain. Nevertheless, their precise rCBF measurements, as validated by radiolabeled microspheres, show the potential of this approach.
The limiting factor for successful implementation of refill kinetics in the brain has been the skull (Postert et al, 1997; Wijnhoud et al, 2008). Because of strong absorption of acoustic signals by the skull, imaging of echo signals from microbubbles circulating in the brain microcirculation requires a high acoustic power. But because ultrasound contrast agents are very fragile, this high power causes them to burst during insonation (Burns, 1996). Therefore, it has not been possible to image the microbubble replenishment phase in real time as in other, more accessible organs.
One attempt to use refill kinetics in humans was undertaken by Seidel et al (2002) in a group of healthy volunteers. This study, however, was limited by triggered imaging sequences and poor temporal resolution because the refill phase could only be imaged with high MI through the temporal bone window. Thus, this promising but technically demanding approach to brain perfusion could not be implemented in stroke patients.
Recent technological advances in ultrasound equipment showing improved sensitivity for detection of microbubbles in the cerebral microcirculation now enable real-time UPI (rt-UPI) through the acoustic bone window in humans (Powers et al, 2009; Seidel and Meairs, 2009). We studied whether rt-UPI is feasible in the setting of acute stroke, and whether refill kinetics may allow identification of ischemic brain tissue.
Materials and methods
Technical Principles of Microbubble Refill Kinetics
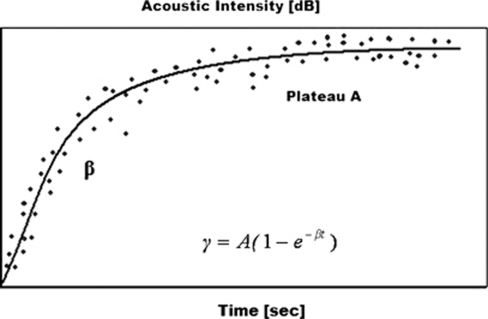
Microbubble tissue replenishment after bubble destruction can be described as an exponential curve with the corresponding equation: 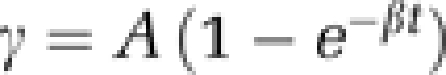 , where β represents the rise rate of acoustic intensity over time and A is the plateau of acoustic intensity reached during the refill kinetic, reflecting the microvascular cross-sectional area (Figure 1). In the original paper of Wei et al (1998), the β parameter was described to be directly related to blood flow velocity, and the plateau of acoustic intensity A related to blood volume. The product of both (A × β) correlated with the blood flow as measured by radiolabeled microspheres (Wei et al, 1998; Rim et al, 2001).
, where β represents the rise rate of acoustic intensity over time and A is the plateau of acoustic intensity reached during the refill kinetic, reflecting the microvascular cross-sectional area (Figure 1). In the original paper of Wei et al (1998), the β parameter was described to be directly related to blood flow velocity, and the plateau of acoustic intensity A related to blood volume. The product of both (A × β) correlated with the blood flow as measured by radiolabeled microspheres (Wei et al, 1998; Rim et al, 2001).
Figure 1.
Exponential function of microbubble replenishment. γ is the acoustic intensity measured at pulse interval t, A is the plateau of acoustic intensity reaching during the refill kinetic, and β is the rise rate of acoustic intensity over time.
Patients
Patients with the clinical syndrome of unilateral acute ischemic stroke in the middle cerebral artery (MCA) territory treated on our Stroke Unit were eligible for the study. All had a baseline computed tomography on admission to rule out intracerebral hemorrhage, which was an exclusion criterion of the study. Patients with insufficient temporal bone windows, defined as failure to depict acoustic signals from standard landmarks such as contralateral skull, mesencephalon, and major arteries of the circle of Willis, were also excluded. Other exclusion criteria were severe heart and lung diseases according to the contraindications of the echo-contrast agent Sonovue (Bracco, Milan, Italy), and evidence of chronic territorial infarction on any hemisphere on admission computed tomography or MRI. Magnetic resonance imaging including diffusion-weighted imaging showing acute cerebral ischemia in the MCA territory was mandatory, with a permitted maximum delay of 8 hours before or after the ultrasound investigation. Clinical status was examined using the National Institute of Health Stroke Scale on admission and three times daily during the initial days of hospitalization. The study was approved by the local ethics committee.
Ultrasound Studies
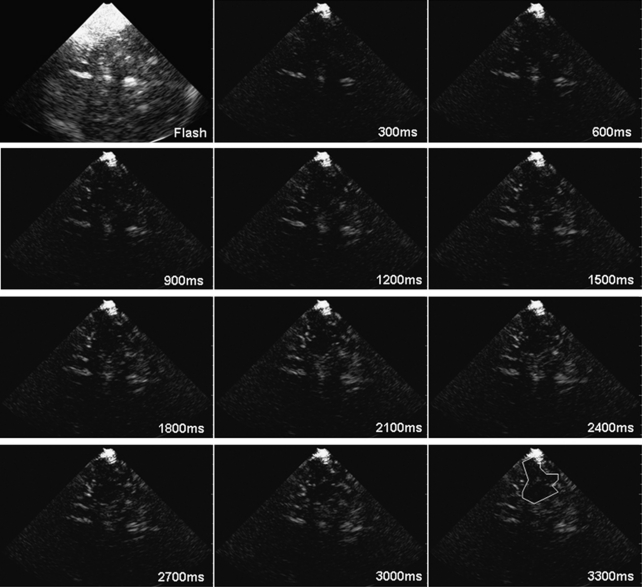
Ultrasound studies were performed using a Philips (Philips Healthcare, Andover, MA, USA) IU22 system with a 1 to 5 MHz dynamic sector transducer for transcranial imaging. Peak systolic flow velocities (PSVs) of brain-supplying vessels including both MCAs were assessed by extracranial and transcranial color-coded duplex ultrasound immediately before rt-UPI studies. For rt-UPI measurements, the transducer was adjusted to a standard axial scan plane tilted to 20° above the mesencephalic plane as described elsewhere (Kern et al, 2005). This allows visualization of brain parenchyma of the MCA territory, including the insular cortex, parts of the temporal and parietal lobe, subcortical white matter and basal ganglia. We used the echo-contrast agent Sonovue for rt-UPI studies. Sonovue is a second-generation ultrasound contrast agent consisting of sulfur hexafluorine gas microbubbles infolded in a phospholipid shell. After intravenous injection of 2.5 mL Sonovue, we first measured the time until the maximum acoustic intensity in the brain tissue was reached, using a low MI of 0.17 and real-time frame rates of 15 Hz (real-time time to peak, rt-TTP). Then we applied 10 repetitive flash frames with a high MI of 1.32 to destroy all microbubbles in the scanning plane. This microbubble destruction is necessary for the refill analysis starting immediately afterwards: again, the replenishment of new microbubbles was measured at a low MI of 0.17, allowing calculation of A and β. The right hemisphere was investigated first in all subjects. The left side was assessed 15 minutes later to allow for disappearance of contrast agent in the brain microcirculation. The same protocol as described above was applied for the examination of the left hemisphere, using a second bolus of 2.5 mL Sonovue. All rt-UPI measurements were digitally stored for offline analysis using the QLab Software Version 5.0 (Philips Healthcare). Regions of interest (ROIs) of ischemic tissue were analyzed and compared with reference regions of similar size and shape taken from the rt-UPI investigation of the contralateral hemisphere. Regions of interest were analyzed by avoiding larger vessels, as inclusion of bigger arteries may influence A and β values (Schlosser et al, 2001). For later statistical analysis, we extracted the values of rt-TTP, A, β, and A × β from the ischemic and the contralateral normal hemisphere. Figure 2 shows an example of microbubble replenishment in a stroke patient: after a high MI flash, the inflow of microbubbles can be observed on successive images obtained with low MI.
Figure 2.
Example of microbubble replenishment in a patient with acute middle cerebral artery stroke: after a flash sequence with high mechanical index (upper left), the inflow of microbubbles can be observed in successive low mechanical index scans. The estimated area of infarction is outlined on the last image of the sequence.
Magnetic Resonance Imaging Studies
Magnetic resonance imaging studies were obtained as soon as possible before or after rt-UPI (mean delay 3.5 hours). Magnetic resonance imaging was performed on a 1.5-T MR System (Magnetom Sonata, Siemens Healthcare, Erlangen, Germany) according to a standardized acute stroke protocol including T1- and T2-weighted sequences, fluid attenuated inversion recovery, diffusion-weighted imaging at b=0, 500, 1,000 s/mm2 with sequential application of three separate diffusion-sensitizing gradients in perpendicular directions, and three-dimensional time of flight magnetic resonance angiography sequence of the intracranial vasculature (circle of Willis). Diffusion-weighted imaging was used for the depiction of localization and size of the infarct in offline analysis of the rt-UPI data. Perfusion dynamic susceptibility contrast imaging (perfusion MRI) was obtained after first pass of a gadolinium contrast bolus through the brain. The contrast agent was injected manually through a large gauge venous cannula in the antecubital vein. The images were postprocessed with a dedicated software package (Nordic Neuro Lab, Bergen, Norway). The measured tissue concentration–time curve was deconvoluted using singular value decomposition with a global arterial input function (∼5 pixels) retrieved from the MCA branches in the hemisphere contralateral to infarction. Perfusion MRI maps such as mean transit time (MTT), rCBF, and regional cerebral blood volume (rCBV) were generated by using an established tracer kinetic model applied to first-pass data. From these maps, ROIs of ischemic tissue were analyzed and compared with reference regions of the contralateral, noninfarcted hemisphere.
Statistical Analysis
All relevant parameters (PSV of MCAs, rt-UPI parameters rt-TTP, A, β, and A × β, as well as perfusion MRI parameters MTT, rCBF, and rCBV as measured from ROIs of ischemic and normal MCA territory) have been analyzed with descriptive statistics (mean, median, s.d., and range). In the following, 95% confidence intervals have been computed to derive the cutoff values between ischemic and normal tissue for each parameter. Significance from unpaired Student's t-tests was used to confirm the discriminating power of the cutoff values. In addition, mean ratios between ischemic and normal tissue were calculated from all relevant parameters. Receiver-operating characteristic (ROC) curves were calculated for rt-UPI and perfusion MRI parameters. The area under the curve (AUC) from the ROC curves was determined for each parameter, including significance testing of AUC. The agreement between mean ratios of β and rCBF, A × β and rCBF, and rt-TTP and MTT were quantified by the coefficient of agreement, which is 1.96 × s.d., where s.d. is the standard deviation of the difference between rt-UPI and MRI parameter. Pearson's correlation was used to evaluate associations between absolute values of rt-UPI and MRI parameters. Statistical analysis was performed using SAS Software Package (Version 9.2, SAS Institute, Cary, NC, USA) and SPSS Statistics Software (Version 17.0, IBM Corporation, Somers, NY, USA).
Results
Twenty-three patients (mean age 70.2±13.2 years, 14 months, 9 weeks) with acute MCA infarction were included (Table 1). Four patients were excluded because of insufficient insonation conditions. The severity of the neurologic deficit as measured with the National Institute of Health Stroke Scale at admission ranged between 0 and 20, with a median score of six. Eleven patients had received intravenous thrombolysis with recombinant tissue plasminogen activator within the 3-hour time window. Diffusion-weighted imaging showed right-sided territorial infarction with cortical involvement in 13 patients and 10 had left-sided infarcts. Seventy eight percent of patients had infarcts affecting between 1/3 and 2/3 of the arterial territory. Peak systolic flow velocities of the MCA were lower ipsilateral to cerebral ischemia (61.3±35.2 versus 89.3±33.1 cm/s; P<0.001). At the time point of ultrasound studies, 11 patients had signs of persistent obstruction of the ipsilateral MCA with PSV reduction of >30% compared with the asymptomatic side. One patient had a focal unilateral increase of PSV (165 cm/s) on the asymptomatic side, suggestive of low-grade MCA stenosis. Extracranial duplex ultrasound revealed a high-grade carotid stenosis ipsilateral to MCA infarction in four patients.
Table 1. rt-UPI parameters from ROIs of ischemic and normal MCA territory.
| Patient | Age (years) | Admission NIHSSS |
Ischemic territory |
Normal territory |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSV MCA (cm/s) | rt-TTP (seconds) | β (1/s) | A | A × β | PSV MCA (cm/s) | rt-TTP (seconds) | β (1/s) | A | A × β | |||
| 1 | 74 | 6 | 80 | 16 | 1.47 | 1.51 | 2.22 | 90 | 11 | 4.81 | 0.28 | 1.35 |
| 2 | 70 | 6 | 60 | 13 | 0.09 | 0.09 | 0.01 | 65 | 10.1 | 1.21 | 5.21 | 6.30 |
| 3 | 62 | 1 | 80 | 9.5 | 1.1 | 0.27 | 0.30 | 165 | 9.5 | 1.07 | 3.25 | 3.48 |
| 4 | 47 | 3 | 40 | 12.5 | 0.53 | 0.52 | 0.28 | 75 | 9.8 | 0.99 | 2.81 | 2.78 |
| 5 | 57 | 17 | 25 | 14 | 0.53 | 1.73 | 0.92 | 55 | 9 | 0.87 | 9.11 | 7.93 |
| 6 | 60 | 14 | 40 | 13 | 0.2 | 2.05 | 0.41 | 75 | 10 | 0.66 | 1.43 | 0.94 |
| 7 | 87 | 12 | 35 | 16 | 0.37 | 0.76 | 0.28 | 70 | 8.5 | 1.07 | 1.49 | 1.59 |
| 8 | 83 | 18 | 0 | 14 | 0.91 | 0.25 | 0.23 | 135 | 8 | 1.03 | 1.38 | 1.42 |
| 9 | 77 | 9 | 60 | 9 | 0.27 | 0.37 | 0.10 | 90 | 8 | 0.76 | 1.07 | 0.81 |
| 10 | 85 | 14 | 110 | 11.5 | 0.65 | 0.65 | 0.42 | 160 | 9 | 0.91 | 4.86 | 4.42 |
| 11 | 61 | 4 | 75 | 13 | 0.14 | 2.2 | 0.31 | 70 | 10 | 0.7 | 1.5 | 1.05 |
| 12 | 84 | 6 | 100 | 8.2 | 0.41 | 0.87 | 0.36 | 90 | 6.5 | 1.23 | 0.85 | 1.05 |
| 13 | 41 | 5 | 50 | 9 | 1.37 | 6.6 | 9.04 | 90 | 8 | 1.49 | 6.99 | 10.42 |
| 14 | 76 | 12 | 60 | 9 | 0.50 | 1.73 | 0.87 | 50 | 8 | 1.57 | 3.44 | 5.40 |
| 15 | 92 | 13 | 50 | 14 | 0.31 | 0.6 | 0.19 | 50 | 14 | 0.65 | 1.17 | 0.76 |
| 16 | 54 | 6 | 0 | 9 | 0.26 | 0.66 | 0.17 | 65 | 6.7 | 0.7 | 0.76 | 0.53 |
| 17 | 66 | 17 | 50 | 7.8 | 0.94 | 1.01 | 0.95 | 90 | 7 | 1.26 | 5.44 | 6.85 |
| 18 | 74 | 2 | 100 | 11.3 | 0.31 | 0.91 | 0.28 | 100 | 10.5 | 0.99 | 0.88 | 0.87 |
| 19 | 86 | 17 | 15 | 15 | 0.3 | 0.22 | 0.07 | 60 | 8.2 | 1.4 | 1.21 | 1.69 |
| 20 | 70 | 1 | 120 | 12 | 1.07 | 6.63 | 7.09 | 150 | 9.2 | 1.64 | 0.93 | 1.53 |
| 21 | 73 | 20 | 130 | 9 | 0.89 | 0.79 | 0.70 | 90 | 7.5 | 1.2 | 1.01 | 1.21 |
| 22 | 66 | 5 | 65 | 9 | 0.36 | 0.48 | 0.17 | 80 | 8.2 | 1.61 | 1.53 | 2.46 |
| 23 | 70 | 3 | 65 | 8.2 | 0.36 | 2.27 | 0.82 | 90 | 7.5 | 0.98 | 3.84 | 3.76 |
| Mean | 70.2 | 9.2 | 61.3 | 11.43 | 0.58 | 1.44 | 1.14 | 89.3 | 8.88 | 1.25 | 2.63 | 2.98 |
| s.d. | 13.2 | 6.1 | 35.3 | 2.67 | 0.40 | 1.76 | 2.25 | 33.1 | 1.66 | 0.83 | 2.31 | 2.70 |
MCA, middle cerebral artery; NIHSSS, National Institute of Health Stroke Scale Score; PSV, peak systolic flow velocity; ROIs, regions of interests; rt-TTP, real-time time to peak; rt-UPI, real-time ultrasound perfusion imaging; s.d., standard deviation.
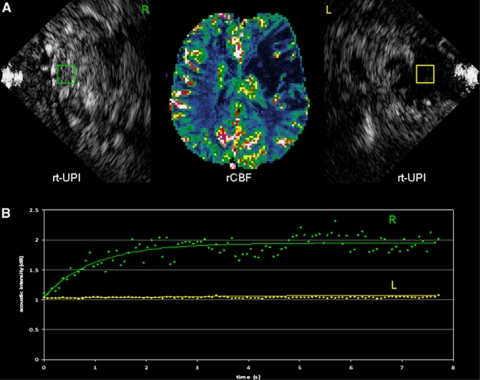
All patients were investigated using rt-UPI within the first 48 hours after stroke onset. None of the patients experienced side effects from the contrast agent. Real-time time to peak measurement and refill analysis of both hemispheres were feasible in all subjects, as well as calculation of the relevant rt-UPI parameters (Table 1). Comparison of ROIs from ischemic tissue and the contralateral hemisphere showed a mean delay of rt-TTP of 2.55 seconds in ischemic tissue (rt-TTP (seconds)=11.43±2.67 versus 8.88±1.66; P<0.001), with a mean ratio of 1.29±0.25 between ischemic and normal tissue. β-values were diminished in ROIs of ischemic tissue compared with the contralateral hemisphere (β (1/s)=0.58±0.40 versus 1.25±0.83; P=0.001). The mean ischemic/normal ratio of the parameter β was 0.48±0.26. A × β was lower in ROIs of the ischemic territory (A × β=1.14±2.25 versus 2.98±2.70; P=0.01; mean ischemic/normal ratio 0.49±0.97). The plateau of acoustic intensity A showed lower values in ROIs of ischemic territory with near-statistical significance (A=1.44±1.75 versus 2.63±2.31; P=0.05), but standard deviations were high and the mean ischemic/normal ratio did not show a difference between the two hemispheres (1.04±1.71). Six patients had even higher A values in the ischemic territory. In these patients, we noticed differences in acoustic attenuation by the temporal bone with better delineation of standard landmarks at this hemisphere. Figure 3 illustrates an example of refill kinetics in a patient with left MCA territory stroke. In this example, a severe reduction of replenishment can be observed in the ROI placed in the ischemic core.
Figure 3.
Example of a patient with acute left middle cerebral artery (MCA) stroke. (A) Perfusion MRI (middle) as displayed on a regional cerebral blood flow (rCBF) map shows severe hypoperfusion in the left MCA territory. Note the striking morphological similarity of the infarct compared with the corresponding ultrasound image from the left hemisphere (image on the right). (B) Real-time ultrasound perfusion imaging (rt-UPI) shows a lower rise rate (β) and plateau (A) of the replenishment curve in a regions of interest (ROI) from the ischemic (yellow) compared with the normal (green) MCA territory.
Analysis of the influence of thrombolytic treatment did not reveal statistical differences between mean side-to-side ratios of sonographic parameters. However, mean PSVs of the MCA were also not different between the two groups, indicating that some patients already had recanalized at the time point of ultrasound examination, and others had persistent MCA obstruction despite thrombolytic treatment. Patients with persistent MCA obstruction had a delayed rt-TTP compared with those having recanalized (mean ischemic/normal ratio 1.42±0.29 versus 1.18±0.14; P=0.02). There were no statistical differences between mean ratios of the other parameters.
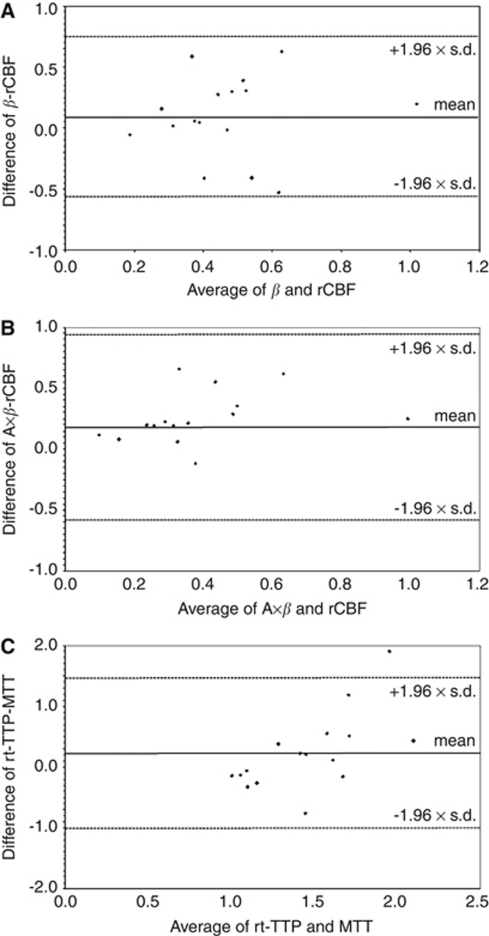
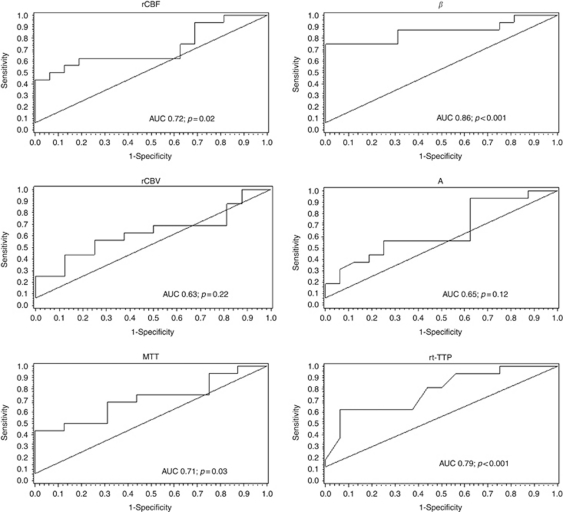
In 16 patients perfusion MRI data sets were obtained. Regional cerebral blood flow was diminished in infarcted tissue compared with ROIs of the normal hemisphere (mean ischemic/normal ratio 0.52±0.26), while MTT was prolonged (mean ischemic/normal ratio 1.57±0.58). Regional cerebral blood volume values were lower in the majority of patients, but the difference was less pronounced (mean ischemic/normal ratio 0.74±0.28). Figure 4 shows Bland–Altman plots of the mean ratios of β and rCBF, A × β and rCBF, and rt-TTP and MTT. Differences for β and rCBF values were on average 0.09±0.33, resulting in a coefficient of repeatability of 0.65 (A × β and rCBF 0.18±0.39, coefficient of repeatability 0.76; rt-TTP and MTT 0.23±0.63, coefficient of repeatability 1.24). Pearson's correlation coefficients showed a positive correlation between rt-TTP and MTT (R=0.51; P=0.003) and a weak negative correlation between rt-TTP and rCBF (R=−0.31; P=0.09). There was no significant correlation between absolute values of the other parameters. ROC analysis (Figure 5) showed higher AUC values of the rt-UPI-derived parameters β (AUC=0.86; P<0.001), A × β (AUC=0.86; P<0.001), and rt-TTP (AUC=0.79; P<0.001) than of rCBF (AUC=0.72; P=0.02) and of MTT (AUC=0.71; P=0.03), indicating a high sensitivity of the ‘refill kinetics' for discriminating ischemic from normal tissue. Area under the curve results were not significant in ROC curves for rCBV (AUC=0.63; P=0.22) and A (AUC=0.65; P=0.12). Optimal cutoff values of rt-UPI parameters for identification of ischemic brain tissue were as follows: rt-TTP >10.1 seconds, β<0.76, A<1.89, and A × β<1.91. Accuracy, sensitivity, specificity, and positive and negative predictive values of rt-UPI parameters are listed in Table 2.
Figure 4.
Bland–Altman plots for mean ischemic/normal ratios of (A) β and regional cerebral blood flow (rCBF), (B) A × β and rCBF, and (C) real-time time to peak (rt-TTP) and mean transit time (MTT).
Figure 5.
Receiver operating characteristics (ROC) curves of perfusion MRI parameters regional cerebral blood flow (rCBF), regional cerebral blood volume (rCBV), and mean transit time (MTT) (left), and of real-time ultrasound perfusion imaging (rt-UPI) parameters β, A, and real-time time to peak (rt-TTP) (right). AUC, area under the curve.
Table 2. Accuracy, sensitivity, specificity, positive (PPV) and negative predictive value (NPV) of rt-UPI-derived parameters.
| Accuracy | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|
| rt-TTP (>10.1 seconds) | 0.72 | 0.77 | 0.68 | 0.63 | 0.81 |
| β (<0.76) | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| A (<1.89) | 0.61 | 0.80 | 0.38 | 0.62 | 0.60 |
| A × β (<1.91) | 0.63 | 0.88 | 0.38 | 0.58 | 0.75 |
rt-TTP, real-time time to peak; rt-UPI, real-time ultrasound perfusion imaging.
Discussion
This is the first study in humans using rt-UPI with a low MI to assess cerebral perfusion through the intact skull. The measurement of refill/replenishment kinetics was feasible in all patients with sufficient insonation conditions (85.1%). The parameters β, A, and A × β identified impaired microbubble replenishment in acute ischemic tissue compared with the normal hemisphere. Similar to MRI-derived rCBF and MTT values, rt-UPI parameters were capable of discriminating ischemic from normal brain tissue and can therefore be used as semiquantitative measures of hemodynamic impairment in acute stroke. In particular, β, A × β, and rt-TTP had a high sensitivity for detecting ischemic brain tissue in the ROC analysis. However, correlation of absolute values failed to show a clear association between rt-UPI and perfusion MRI parameters, except for rt-TTP and MTT. Although A values were diminished in ischemic tissue with near-statistical significance, the standard deviation was high, and this parameter showed a relatively low AUC in the ROC analysis without reaching significance. This observation can be explained by the differences of acoustic attenuation of the skull, which may reduce the reliability of an interindividual comparison of A values. β values and A × β, however, appear less dependent on external variables.
The performance of real-time refill kinetics is relatively simple as compared with previous ultrasound methods for evaluation of brain tissue perfusion. Data collection, for example, takes only ∼10 seconds after optimal positioning of the transducer. Analysis of data is possible online and involves selection of one or multiple ROIs. Consequent calculation and display of refill kinetics are practically instantaneous, thus allowing immediate bedside interpretation of results. These features reflect important advantages over first-generation techniques for UPI, which were limited by relatively difficult and operator-dependent data acquisition, tedious data transfer, and off-line analysis. Thus, the real-time nature of this approach should enable a new dimension in bedside monitoring of brain perfusion with ultrasound.
Another significant advantage of rt-UPI over previous ultrasound techniques for evaluation of brain tissue perfusion is that this approach avoids the shadowing effect, a significant problem associated with high MI imaging. Because of the high acoustic intensities that are emitted by bursting bubbles when they are destroyed by high MI imaging, bubbles that are ‘behind' the emitting bubbles (further away from the ultrasound transducer) are ‘shadowed' by this effect and thus obscured from data analysis. Thus, areas of tissue that are shadowed may not be available for analysis of tissue perfusion. The problem of shadowing is eliminated with low MI imaging, because bubbles are not destroyed with such low acoustic pressure.
The results of this first report on real-time brain perfusion analysis in stroke patients remain semiquantitative, and a subanalysis could not show consistent differences of the parameters with respect to therapeutic recanalization. This is likely because of the variation in the plateau level of microbubble replenishment, as can be expected as a consequence of different attenuations of the skull between patients. However, this should not be considered as an inherent, unsolvable problem that limits the future application of rt-UPI. New ultrasound technology is under development, which should be capable of providing good estimates of skull attenuation. This in turn may allow improved analysis of the plateau level A in refill kinetics, a necessary parameter for calculation of rCBF with UPI. Thus, our study showing that rt-UPI is indeed feasible in stroke patients and is capable of providing real-time information on refill kinetics to differentiate between normal and ischemic brain tissue, provides good support for further work to optimize the quantitative potentials of rt-UPI. In this context, it will also be of great interest to evaluate the potential of this method for characterization of therapeutic effects in a larger number of patients treated with tissue plasminogen activator.
One requirement for quantitative assessment of refill kinetics is a steady-state microbubble concentration. In this study, we did not use a constant infusion of contrast agent, but rather delivered the microbubbles in bolus manner. Since a relatively stable plateau level is maintained during the first minute after bolus injection of Sonovue, measurements of the acoustic intensity during this time in the same imaging planes of both hemispheres are comparable (Powers et al, 2009). However, to exploit the full potential of refill kinetics in multiplanar analysis, which is now theoretically possible with rt-UPI, a steady-state concentration will be necessary. Other limitations are the relatively small number of included patients, and the lack of intraobserver reliability and interobserver variability studies, which would have required repeated injections of contrast agent.
We propose that real-time, low MI imaging with analysis of refill kinetics is the ideal approach for UPI because it offers a far better temporal resolution than all other modalities that have been described previously. It has a larger potential than standard bolus kinetic analysis, which is limited to triggered image sequences of 1 Hz or less because of bubble destruction by high MI insonation. Further investigations should be undertaken to prove the superiority of this method compared with conventional UPI approaches for assessment of bolus kinetics. We believe that the use of this real-time, low MI technique will improve the diagnostic confidence of UPI in acute stroke and other conditions of impaired cerebral perfusion. In particular, it may be performed for monitoring of reperfusion during the acute phase of stroke after thrombolytic treatment and has the great advantage of being possible at bedside on stroke units and intensive care units.
Acknowledgments
The authors acknowledge technical support by Jeff Powers, PhD, Philips Ultrasound, Bothell, USA.
The authors declare no conflict of interest.
References
- Burns PN. Harmonic imaging with ultrasound contrast agents. Clin Radiol. 1996;51 (Suppl 1:50–55. [PubMed] [Google Scholar]
- Elhendy A, Porter TR. Assessment of myocardial perfusion with real-time myocardial contrast echocardiography: methodology and clinical applications. J Nucl Cardiol. 2005;12:582–590. doi: 10.1016/j.nuclcard.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Eyding J, Krogias C, Schöllhammer M, Eyding D, Wilkening W, Meves S, Schröder A, Przuntek H, Postert T. Contrast-enhanced ultrasonic parametric perfusion imaging detects dysfunctional tissue at risk in acute MCA stroke. J Cereb Blood Flow Metab. 2006;26:576–582. doi: 10.1038/sj.jcbfm.9600216. [DOI] [PubMed] [Google Scholar]
- Federlein J, Postert T, Meves S, Weber S, Przuntek H, Büttner T. Ultrasonic evaluation of pathological brain perfusion in acute stroke using second harmonic imaging. J Neurol Neurosurg Psychiatry. 2000;69:616–622. doi: 10.1136/jnnp.69.5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Kracht L, Grond M, Rudolf J, Bauer B, Wienhard K, Pawlik G. Early [(11)C]Flumazenil/H(2)O positron emission tomography predicts irreversible ischemic cortical damage in stroke patients receiving acute thrombolytic therapy. Stroke. 2000;31:366–369. doi: 10.1161/01.str.31.2.366. [DOI] [PubMed] [Google Scholar]
- Hjort N, Butcher K, Davis SM, Kidwell CS, Koroshetz WJ, Rother J, Schellinger PD, Warach S, Ostergaard L. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke. 2005;36:388–397. doi: 10.1161/01.STR.0000152268.47919.be. [DOI] [PubMed] [Google Scholar]
- Kern R, Perren F, Kreisel S, Szabo K, Hennerici M, Meairs S. Multiplanar transcranial ultrasound imaging: standards, landmarks and correlation with magnetic resonance imaging. Ultrasound Med Biol. 2005;31:311–315. doi: 10.1016/j.ultrasmedbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Kern R, Perren F, Schoeneberger K, Gass A, Hennerici M, Meairs S. Ultrasound microbubble destruction imaging in acute middle cerebral artery stroke. Stroke. 2004;35:1665–1670. doi: 10.1161/01.STR.0000129332.10721.7e. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- Krogias C, Postert T, Meves S, Wilkening W, Przuntek H, Eyding J. Semiquantitative analysis of ultrasonic cerebral perfusion imaging. Ultrasound Med Biol. 2005;31:1007–1012. doi: 10.1016/j.ultrasmedbio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Meairs S, Daffertshofer M, Neff W, Eschenfelder C, Hennerici M. Pulse-inversion contrast harmonic imaging: ultrasonographic assessment of cerebral perfusion. Lancet. 2000;355:550–551. doi: 10.1016/S0140-6736(99)04361-5. [DOI] [PubMed] [Google Scholar]
- Olivot JM, Mlynash M, Zaharchuk G, Straka M, Bammer R, Schwartz N, Lansberg MG, Moseley ME, Albers GW. Perfusion MRI (Tmax and MTT) correlation with xenon CT cerebral blood flow in stroke patients. Neurology. 2009;72:1140–1145. doi: 10.1212/01.wnl.0000345372.49233.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postert T, Federlein J, Przuntek H, Buttner T. Insufficient and absent acoustic temporal bone window: potential and limitations of transcranial contrast-enhanced color-coded sonography and contrast-enhanced power-based sonography. Ultrasound Med Biol. 1997;23:857–862. doi: 10.1016/s0301-5629(97)00047-1. [DOI] [PubMed] [Google Scholar]
- Powers J, Averkiou M, Bruce M. Principles of cerebral ultrasound contrast imaging. Cerebrovasc Dis. 2009;27 (Suppl 2:14–24. doi: 10.1159/000203123. [DOI] [PubMed] [Google Scholar]
- Rim SJ, Leong-Poi H, Lindner JR, Couture D, Ellegala D, Mason H, Durieux M, Kassel NF, Kaul S. Quantification of cerebral perfusion with ‘Real-Time' contrast-enhanced ultrasound. Circulation. 2001;104:2582–2587. doi: 10.1161/hc4601.099400. [DOI] [PubMed] [Google Scholar]
- Schlosser T, Pohl C, Veltmann C, Lohmaier S, Goenechea J, Ehlgen A, Köster J, Bimmel D, Kuntz-Hehner S, Becher H, Tiemann K. Feasibility of the flash-replenishment concept in renal tissue: whichparameters affect the assessment of the contrast replenishment. Ultrasound Med Biol. 2001;27:937–944. doi: 10.1016/s0301-5629(01)00397-0. [DOI] [PubMed] [Google Scholar]
- Seidel G, Algermissen C, Christoph A, Claassen L, Vidal-Langwasser M, Katzer T. Harmonic imaging of the human brain. Visualization of brain perfusion with ultrasound. Stroke. 2000;31:151–154. doi: 10.1161/01.str.31.1.151. [DOI] [PubMed] [Google Scholar]
- Seidel G, Meairs S. Ultrasound contrast agents in ischemic stroke. Cerebrovasc Dis. 2009;27 (Suppl 2:25–39. doi: 10.1159/000203124. [DOI] [PubMed] [Google Scholar]
- Seidel G, Meyer K, Metzler V, Toth D, Vida-Langwasser M, Aach T. Human cerebral perfusion analysis with ultrasound contrast agent constant infusion: a pilot study on healthy volunteers. Ultrasound Med Biol. 2002;28:183–189. doi: 10.1016/s0301-5629(01)00501-4. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG, Barrett EJ. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes. 2002;51:42–48. doi: 10.2337/diabetes.51.1.42. [DOI] [PubMed] [Google Scholar]
- Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered asa constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol. 2001;37:1135–1140. doi: 10.1016/s0735-1097(00)01210-9. [DOI] [PubMed] [Google Scholar]
- Wijnhoud AD, Franckena M, van der Lugt A, Koudstaal PJ, Dippel ED. Inadequate acoustical temporal bone window in patients with a transient ischemic attack or minor stroke: role of skull thickness and bone density. Ultrasound Med Biol. 2008;34:923–929. doi: 10.1016/j.ultrasmedbio.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel JF, Nariai T, Zaharchuk G, Caille JM, Dousset V, Yonas H. Comparative overview of brain perfusion imaging techniques. Stroke. 2005;36:e83–e99. doi: 10.1161/01.STR.0000177884.72657.8b. [DOI] [PubMed] [Google Scholar]