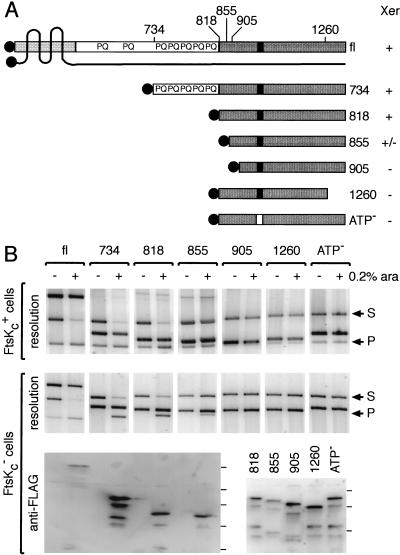
Figure 1.
Minimal region of FtsK required for Xer recombination. (A) FtsK derivatives used in this study. The Nterminal domain of FtsK, domain 1, is represented by a lightly shaded box, and the four putative transmembrane regions are shown as black lines crossing the protein. The extreme N terminus points toward the cytoplasm, as well as domain 2 and 3 of the protein. The C-terminal domain, domain 3, is shown as a dark box, and the putative nucleotide binding site as a black rectangle. The Flag peptide is shown as shaded circles. The N-terminal (734, 818, 855, 905) and C-terminal (1260) amino acid of each protein are indicated on top of the full-length protein (fl). The relative ability of each protein to support Xer recombination on plasmids is indicated by +/− signs on the right of the diagram. (B) Xer recombination between plasmid-borne dif sites in cells expressing FtsK deletions. FtsKc+ (DS941) and FtsKc− (DS9041) cells (as shown to the left of the gels) were transformed with the different ftsK expression vectors, as indicated along the top of the gels. Plasmid-containing cells were grown for 5 h in arabinose (+, 0.2% ara) or in glucose (−). Recombination between the two dif sites carried by the substrate (S) leads to the formation of two smaller product circles, of which one can replicate (P). The substrate and product bands are indicated by arrows to the right of the gels. The third band present in each lane is the ftsK expression vector. Expression of the FtsK derivatives was verified by Western blot analysis after resolution of the proteins on a 6% (fl, 734, 818, 855) or a 8% (818, 855, 905, 1260, ATP−) SDS-PAGE. Complete repression (−) was verified for fl, 734, 818, and 855. The position of the protein molecular weight markers are indicated on the right of each blot and correspond from bottom to top to 32, 47, 67, 83, and 175 kD. The relatively low signal with fl may reflect a poorer transfer to the membrane.