Abstract
Despite the use of immunosuppressives mainly influencing T and B cell responses, the prevalence of the bronchiolitis obliterans syndrome (BOS) after lung transplantation is high. Mannose-binding lectin (MBL) is a pattern recognition molecule of complement and an important component of the innate immunity. MBL is associated with rejection, infection and survival in other solid organ transplantations. In this study the relation between functional MBL levels and cytomegalovirus (CMV) reactivations and the development of BOS and survival after lung transplantation was investigated. MBL levels were measured in 85 patients before and in 57 of these patients after lung transplantation. The relation of MBL on survival, CMV reactivation and the development of BOS were investigated with Kaplan–Meier (log-rank) survival analysis. MBL levels decreased on average by 20% (P < 0·001) after transplantation and eventually returned to pretransplant levels. Fourteen of the 85 patients had deficient pretransplant MBL levels and these patients had a tendency towards a better survival compared to those with normal MBL levels (P = 0·08). Although no correlation was found between MBL deficiency and the development of BOS, more CMV reactivations occurred in recipients with deficient versus normal levels of MBL (P = 0·03). Our results suggest that MBL deficiency is associated with CMV reactivations and a longer overall survival, but not with the development of BOS.
Keywords: BOS, CMV, lung, MBL, transplantation
Introduction
Lung transplantation is an accepted therapy for end-stage lung diseases. Although survival after lung transplantation has improved significantly in the last decades, a 5-year survival of approximately 50% remains poor [1]. This is due mainly to the development of the bronchiolitis obliterans syndrome (BOS), which is clinically diagnosed by a decrease in lung function [2]. BOS is acknowledged to be an immune-driven response. Nevertheless, despite widely used immunosuppressives such as calcineurin inhibitors, prednisone, mycophenolate acid derivates and mTOR inhibitors, all mainly influencing T and B cell responses, the occurrence of chronic rejection is high, suggesting that the innate immune system may contribute at least in part to the development of BOS.
The complement system plays an important role in the innate immune system and can be activated by three different pathways: the classical, the alternative and the lectin pathway. The initiator of the lectin pathway is the mannose-binding lectin (MBL), which binds to carbohydrate residues including mannose, fucose and N-acetylglucosamine on the surfaces of microorganisms such as viruses, bacteria and fungi. After binding to MBL, the MBL-associated serine protease (MASP-2) is activated and leads to cleavage of C4 and C2 followed by formation of C4b2a. The latter acts as a C3 convertase and further on in the cascade the microorganism is opsonized and phagocytosed. MBL deficiencies are associated with an enlarged vulnerability to bacterial infections as seen in allogeneic stem cell transplantation, after liver and simultaneous pancreas–kidney transplantation and in neutropenic patients after chemotherapy [3–6]. In addition, MBL deficiency is a risk factor for cytomegalovirus (CMV) infection after kidney transplantation [7,8].
Interestingly, MBL levels have been correlated with graft survival. In a follow-up of more than 10 years, MBL deficiency was correlated with improved graft survival in 266 kidney and 99 pancreas–transplant recipients, respectively [9,10]. In heart transplantation MBL deficiency was associated with chronic rejection in a study following 38 heart transplant recipients, but these results could not be confirmed in 90 heart transplant recipients [11,12]. Perhaps the permissive cold ischaemia time is of influence, which is much longer in kidney and pancreas–kidney transplantation compared to heart transplantation. MBL deficiency prevents complement activation and it has been suggested that MBL deficiency may be beneficial in ischaemic injury [13,14]. In lung transplantation an association was found between donor lungs with the MBL-encoding haplotype LXPA and BOS [15]. This haplotype is associated with MBL deficiency [16–18]. The influence of MBL genotype from donor lungs on the development of BOS, instead of the MBL genotype of the recipients, is remarkable because MBL is produced mainly in the liver. This finding suggests that, in lung transplant recipients, production of extrahepatic MBL production is relevant to bronchiolar damage after lung transplantation.
The relation between circulating levels of MBL and clinical outcome after lung transplantation has not yet been studied. Therefore, the aim of our study was to examine the relation between functional MBL levels in recipients and the development of BOS and/or (re)occurrence of CMV infection.
Materials and methods
Subjects
Patients who underwent lung transplantation at the Heart Lung Center in Utrecht between September 2001 and November 2008 were included in this study. Beginning in September 2003, all patients were included into a research protocol whereby sera and clinical information were collected regularly. Several hours prior to transplantation serum was collected and stored at −80°C. According to the standard International Society for Heart and Lung Transplantation (ISHLT) criteria, BOS was defined as a decline of the forced expiratory volume in 1 s (FEV1) of more than 20% in the absence of infection or other aetiology [2]. To avoid the bias of postoperative complications in the diagnosis of BOS, we excluded patients who died during the period from transplantation to 3 months after transplantation. The immunosuppressive regimen consisted of anti-CD25 induction, tacrolimus, mycophenolate mofetil and prednisone. Patients at risk for CMV reactivation, defined as a CMV-seropositive donor or recipient, were treated with valganciclovir for 6 months post-transplantation. The study was approved by the medical ethical committee and informed consent was obtained from each patient.
MBL analysis
Serum was collected prior to transplantation, stored at −80°C and thawed at a later time-point for quantitative determination of functional MBL levels performed by a commercially available enzyme immunoassay (Wieslab™ COMPL MP320, Land, Sweden) [19,20]. This assay evaluates all three pathways of complement activation. In brief for MBL, wells were precoated with mannan. Sera were diluted and pipetted in these wells. After washing, the wells were incubated with antibodies against C9 and processed further according the manufacturer's instructions. Absorbance values were read at 405 nm. The negative control is given a value of 0%, the positive control is given a value of 100%, and subsequently the values of the sera were expressed as a percentage of the positive control. This assay was standardized and validated in sera from 120 healthy donors in three laboratories. In this standardization and validation study, the mean value of complement activity was calculated from these 120 donors. There is a large variation of MBL levels with a distribution skewed to the left. The threshold level for MBL deficiency was set arbitrarily at 10% of signal in the enzyme-linked immunosorbent assay (ELISA) corresponding to MBL levels below 300 ng/ml. Samples were not frozen and thawed more than once. No MBL levels were measured in the donor.
CMV (re)activation
CMV serostatus of patient and donor were determined by ELISA (VIDAS-biomerieux, Marcy L'Etoile, France). Post-transplant monitoring for CMV (re)activation was performed by polymerase chain reaction (PCR). We used the PCR (Taqman) whereby a serological (re)activation was defined as a successive assay detecting more than 400 copies/ml in serum. After October 2003 we increased our volume and used 3 ml serum instead of 1 ml and therefore our threshold for a successive assay could be lowered to 50 CMV copies/ml instead of 400 copies/ml. Not all of our CMV reactivations needed treatment with valganciclovir. Patients with a clinical CMV (re)activation, defined as more than 1000 copies/ml, were treated with valganciclovir and reducing immune suppression. Duration and intensity of valganciclovir depended upon clinic and viral load. None of the MBL-deficient patients were receiving immunosuppressives.
Statistical analysis
Analysis was based on the sera availability. A power analysis was performed to detect the number of transplant procedures needed to be included to detect a difference in 5-year graft survival of 30% between MBL-deficient recipients versus MBL-sufficient recipients. Based on literature about both CMV infections and MBL values in kidney transplantation and graft survival in lung transplantation, 5-year graft survival was estimated at between 45 and 50% [1,21]. With a two-sided risk of 5%, a power of 80% and the estimation that 33% of the population had low MBL values, we needed 82 transplant procedures. The Kruskal–Wallis test was used to compare MBL levels between native lung diseases. The Wilcoxon signed-rank test was performed in order to evaluate MBL levels before and after lung transplantation. Post-MBL values were compared by a multivariate analysis of covariance (ancova), adjusted for gender, type of transplantation, underlying disease and the development of BOS. The statistical significance of MBL levels in relation to survival, CMV reactivation and the BOS-free period was analysed with a log-rank test in the Kaplan–Meier curve. Fisher's exact test was used to compare frequencies. P < 0·05 was considered statistically significant.
Results
In the period from September 2001 to November 2008, 133 lung transplant procedures were performed. Thirty-two patients were transplanted before September 2003 and pretransplantation sera, but no post-transplantation sera, were available from 13 of these patients. Since September 2003, 101 patients were transplanted and in this group 17 patients died within 3 months after transplantation and three patients were transferred to other transplantation centres, and therefore excluded. Pre- and post-transplantation sera were missing from nine patients. Six patients died before the second serum sample, and post-transplantation sera were missing from six patients. Sera from 85 patients were collected prior to transplantation; we collected serum after transplantation from 72 patients and both prior and after transplantation from 57 patients. Twenty-one (25%) of the 85 patients included in the study developed BOS during their follow-up. Two patients underwent retransplantation due to graft failure. The characteristics of this study cohort are shown in Table 1.
Table 1.
Characteristics of study group.
| Pretransplantation | MBL < 10% | MBL ≥ 10% | |
|---|---|---|---|
| n = 85 | n = 14 | n = 71 | |
| MBL values (in %) | 95% CI (63·5–84·7) | ||
| Mean follow-up (in months) | 55 (5–102) | 55 (5–102) | 51 (30–102) |
| Age (in years) | 45 (16–64) | 48 (17–62) | 50 (16–64) |
| Gender male/female | 42/43 | 8/6 | 34/37 |
| Bilateral/unilateral | 69/16 | 9/5 | 60/11 |
| Native disease | |||
| CF | 23 (27%) | 4 (29%) | 21 (30%) |
| Emphysema | 38 (45%) | 9 (64%) | 29 (40%) |
| Fibrotic disease | 24 (28%) | 1 (7%) | 21 (30%) |
| CMV IgG status | |||
| Donor+/recipient– | 1 | 19 | |
| Donor+/recipient+ | 3 | 7 | |
| Donor–/recipient+ | 8 | 16 | |
| Donor–/recipient– | 2 | 17 | |
| Donor?/recipient+ | – | 12 | |
| Copies in CMV reactivation, mean (range) | 2651 (range 50–2651) | 2558 (range 0–10 724) | |
| HLA class I mismatch | 3·1 ± 0·8 | 3·2 ± 0·8 | |
| HLA class II mismatch, mean (s.d.) | 1·6 ± 0·5 | 1·7 ± 0·5 | |
| BOS | 21 | 6 | 15 |
MBL: mannose-binding lectin; CF: cystic fibrosis; CMV: cytomegalovirus; IgG: immunoglobulin G; Donor?, means that the CMV status of the donor was not determined; CMV reactivation: detecting more than 50 copies/ml in serum; HLA: human leucocyte antigen; BOS: bronchiolitis obliterans syndrome; CI: confidence interval; s.d.: standard deviation.
MBL values before and after lung transplantation
Prior to lung transplantation, 14 of 85 patients had MBL values less than 10% (MBL-deficient), corresponding with MBL levels below 300 ng/ml. No significant differences in recipients' age, human leucocyte antigen (HLA)-mismatches, sputum cultures shortly before transplantation, C-reactive protein (CRP) or gender distribution were observed between the two groups with sufficient and MBL-deficient values. Of the 85 patients, 24 patients suffered from fibrotic disease with mean MBL values of 92·5% [95% confidence interval (CI): 71·6–113·4%], 38 from emphysema with mean MBL values of 63·5 % (95% CI: 47·2–79·7%) and 23 from CF with mean MBL values of 73·8% (95% CI: 54·5–93·3%). Analysis of MBL values showed no association between pretransplant MBL values and native lung diseases.
To analyse whether the transplantation procedure in combination with immunosuppressive therapy had an effect on MBL levels, MBL values were measured in 57 patients before and ±20 months after transplantation. As shown in Fig. 1a, MBL values decreased significantly after transplantation (P < 0·0001) (95% CI: 9·9–23·8). On average, MBL levels were reduced by 20% after transplantation. Although MBL values decreased in most patients after transplantation, an increase in MBL values was detected in 12 patients after transplantation. No correlation was found between an increase in MBL values and sputum cultures before transplantations, native disease or CMV copies after transplantation.
Fig. 1.
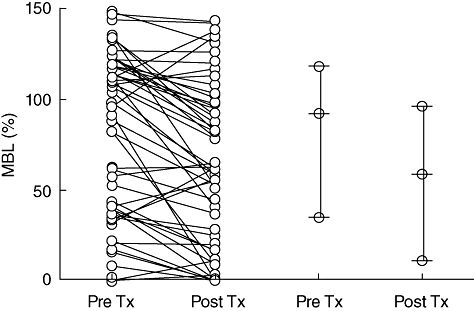
Serum mannose-binding lectin (MBL) values were measured in 57 lung transplant recipients before and 20 months after lung transplantation. Each circle represents the MBL measured in one serum. The decrease in MBL values was significant and medians and 25–75% intervals are depicted pre- and post-lung transplantation. In the 45 patients who showed a decrease in MBL values after transplantation, no differences in native lung diseases were detected. Twelve patients (six cystic fibrosis and six emphysema) demonstrated an increase in MBL values after transplantation.
MBL values and CMV reactivation
In 24 of the 71 patients with normal pretransplant MBL values (33%), CMV copies were detected during follow-up, which was not significantly different compared to seven of the 14 patients with low MBL values (50%) (P = 0·30). None of the CMV reactivations occurred while patients received valganciclovir prophylaxis. It is remarkable that CMV copies were detected in 50% of patients with low pretransplant MBL levels, as only one patient was considered a high-risk patient for CMV reactivation (CMV serostatus donor+/recipient-). This may suggest that patients with pretransplant MBL deficiency have a high risk of CMV reactivation. Therefore we analysed CMV reactivation in recipients with a CMV immunoglobulin G (IgG)-positive status. Prior to transplantation, 35 of 71 patients (49%) with normal MBL levels and 11 of 14 patients (79%) with MBL-deficient levels had IgG antibodies against CMV (P = not significant). In the CMV seropositive patients, CMV reactivation after transplantation was detected in nine of 35 recipients with normal pretransplant MBL levels (26%), compared to seven of the 11 with MBL-deficient levels (64%) (P = 0·03). Kaplan–Meier analysis showed a non-significant tendency towards more CMV reactivations in patients with pretransplant MBL-deficient levels and a positive IgG status (Fig. 2a) [P = 0·06; hazard ratio (HR) 0·3; 95% CI: 0·1–1·0].
Fig. 2.
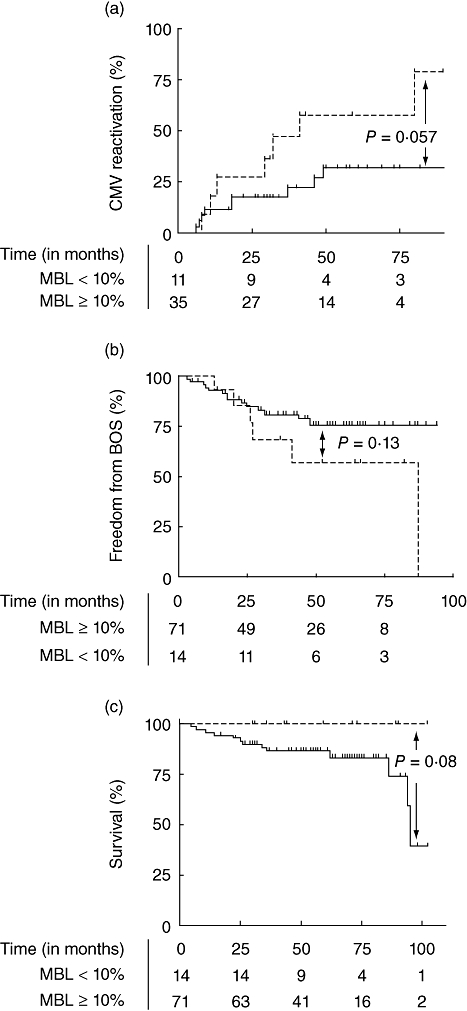
(a) Kaplan–Meier analysis of cytomegalovirus (CMV) reactivation showed a trend towards more CMV reactivations in recipients with deficient pretransplant mannose-binding lectin (MBL) values (solid line). Patients with normal pretransplant MBL values are depicted with a dashed line. CMV reactivation was defined as a successive assay in the polymerase chain reaction after transplantation in those patients who had a seropositive CMV status before transplantation. (b) Kaplan–Meier analysis of freedom from bronchiolitis obliterans syndrome (BOS) showed no significant difference in recipients with deficient pretransplant MBL values (solid line) compared to normal pretransplant MBL values (dashed line). (c) Kaplan–Meier analysis of survival showed a trend towards a better survival in recipients with deficient pretransplant MBL values (solid line) compared to normal pretransplant MBL values (dashed line).
Post-transplant sera were available in 72 patients, but no influence of post-transplantation MBL on CMV reactivation was detected (P = 0·4).
MBL values, BOS and survival
Thirteen patients died during the study, six of whom were diagnosed with BOS. Fourteen of the 71 patients with normal pretransplant MBL values (20%) developed BOS, compared to seven of the 14 patients in the group with MBL-deficient values (50%) (P = 0·02). In a Kaplan–Meier analysis no correlation was found between pretransplant MBL values and the development of BOS (Fig. 2b) (P = 0·13; HR 0·4; 95% CI 0·1–1·3). However, there was a non-significant tendency towards a better survival in patients with MBL-deficient levels prior to transplantation (Fig. 2c) (P = 0·08; HR 3·4; 95% CI 0·9–4·0).
Post-transplant sera were available from 72 patients, but no influence of post-transplant MBL values on survival (P = 0·82; HR 1·32; 95% CI: 0·3–5·6), nor was BOS detected (P = 0·17; HR 0·4; 95% CI: 0·1–1·4).
Analysing the 57 patients for whom pre- and post-transplantation sera were available showed that no patients with MBL deficiency died, whereas 83% of patients with normal MBL survived (P = 0·25; HR 0·4; 95% CI: 0·4–24·9). In this group, MBL-deficient patients had a freedom from BOS of 53% versus 78% in normal MBL patients (P = 0·32; HR 2·2; 95% CI: 0·5–11·0).
Discussion
Long-term survival after lung transplantation is poor, due mainly to the development of BOS. Although BOS is caused largely by alloimmunity, there is hardly any clinical improvement to augmented therapy compromising the adaptive immune system. Therefore, we presume that the innate immunity also plays a role in the development of BOS. Earlier studies suggested an influence of MBL levels on infections and survival in solid organ transplants [8–10,15]. In this study we examined the relation between functional MBL values and clinical outcome [CMV reactivation and (graft) survival] after lung transplantation.
Because immunosuppressive therapy was tapered in the first year after transplantation and maintained thereafter, we presumed that MBL levels would be stable 20 months after transplantation. However, MBL levels dropped in this time-frame after transplantation by 20%, and MBL values increased later and reached values comparable to those before transplantation. Recently, in accordance with our results, reduced MBL levels in both serum and bronchoalveolar lung fluid have been demonstrated in lung transplant recipients [22]. Because the transplanted lung is exposed continuously to pathogens in the air (e.g. pollution, viruses) there is persistent epithelial injury and repair, and an increased apoptosis in airway epithelial cells [23]. Apoptotic epithelial cells are removed by phagocytotic cells; this process is called ‘efferocytosis’. MBL influences efferocytosis [24]. It is possible that MBL levels in lung transplant recipients are reduced because of consumption due to persistent efferocytosis. In addition, we cannot exclude the influence of drugs. Due possibly to the immunosuppressives which are cleared mainly by the liver, MBL synthesis is compromised by the liver. For liver transplantation it was demonstrated that MBL levels increase after transplantation and are stable for more than 1 year [4,25]. The immunosuppressive regimen in liver transplantation is mild compared to lung transplantation, which may explain the differences in post-transplant MBL levels found between these types of organ transplantation. In the aforementioned studies it was also shown that MBL is produced mainly by the transplanted liver [4]. Analysis on extrahepatic production of MBL showed mRNA expression in the small intestine and testis, but not in the lung [26]. Munster et al. demonstrated that donor haplotype LXPA correlated with a superior lung transplant outcome, but only during an immunosuppressive regimen of cyclosporine, azathioprine and prednisone. After introducing the new immunosuppressive protocol, the influence of MBL haplotype on graft survival disappeared. As the study by Munster et al. was performed in the Netherlands and the majority of recipients and donors in our cohort are from the Netherlands and matched according the same Eurotransplant algorithms, we presume that the distribution of the MBL haplotypes is similar to Munster et al.'s cohort. In addition, their new immunosuppressive protocol is similar to ours, with the exception that we use mycophenolate mofetil instead of azathioprine. Nevertheless, MBL has been found in bronchoalveolar lavage (BAL) fluid, although only during inflammation, which may be caused by local production of activated alveolar cells or by ‘leakage’ from the serum [27]. Nevertheless, it seems unlikely that small amounts of MBL produced locally in the lung contribute significantly to the amount present in the circulation. Until the present no (functional) RNA fragments in the lung have been described in the database of Online Mendelian Inheritance in Man (OMIM) [28].
MBL deficiency is associated with viral infections [29–31]. The results of our study demonstrated more CMV reactivations in patients with pretransplant MBL-deficient values. This is in line with the results from Manuel et al., who studied plasma MBL levels in 16 kidney transplant patients with a high-risk CMV status (donor-positive/receptor negative) and reported an association with MBL status and CMV reactivation [7]. In stem cell transplantation, an association was also found between MBL levels and CMV infection/reactivation in 131 patients [32]. Although we observed a constantly higher amount of CMV reactivations in the group with MBL-deficient levels, this did not reach statistical significance in a Kaplan–Meier analysis (P = 0·06). In our daily clinical practice we use CMV reactivation as a tool to detect an over-suppressed immune system, and if CMV copies are detected we reduce immunosuppression. During CMV prophylaxis no CMV reactivations occurred. In addition, 13 patients, of whom six had BOS, died during the study, indicating that survival and BOS are not linked in this study. This might explain why low MBL values could be associated with a better survival, but not with BOS. Our results on the non-significant correlation between MBL levels and CMV reactivation/development of BOS should be interpreted with caution, as we have a relatively small number of patients.
Not only MBL deficiency, but also the development of BOS and survival, are associated with viral, bacterial and fungal infections [33–35]. Unfortunately, in our follow-up of lung transplant recipients no surveillance bronchoscopies, sputum cultures or surveillance viral swabs are implemented and therefore we could not explore the association of MBL values and fungal, mycobacterium and common viral infections. The association between CMV infections and BOS is disputed. Some studies showed an association, but others do not [36–38]. We found an association between MBL deficiency and the development of BOS (P = 0·02), although this did not reach statistical significance in a Kaplan–Meier analysis. This is in line with the results of Munster et al., who no longer found a correlation between MBL and the development of BOS after switching to an immunosuppressive regimen consisting of tacrolimus, mycophenolate mofetil and prednisone [15]. Because of the use of azithromycin, some patients with BOS have a reversible or a lesser decreasing lung function, and this may have an impact on survival after lung transplantation [39–41]. Patients with normal MBL values before transplantation had a non-significant tendency towards a worse survival, suggesting that the contribution of MBL in local complement activation is related to graft survival.
A limitation of our study may be that in our study only 14 of 85 patients had MBL-deficient levels (instead of the expected one-third). This could have led to the non-significant result, and a type II error.
In conclusion, the findings of the present study suggest that MBL deficiency is associated with CMV reactivations and longer survival, but not with the development of BOS. Larger studies are needed to confirm these findings further.
Acknowledgments
We thank Mrs Coby v.d. Velde for her excellent technical assistance.
Disclosure
The authors declare that they have no conflict of interest related to the publication of this manuscript.
References
- 1.Christie JD, Edwards LB, Aurora P, et al. Registry of the international society for heart and lung transplantation: Twenty-Fifth Official Adult Lung and Heart/Lung Transplantation Report – 2008. J Heart Lung Transplant. 2008;27:957–69. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–4. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 3.Mullighan CG, Heatley SL, Danner S, et al. Mannose-binding lectin status is associated with risk of major infection following myeloablative sibling allogeneic hematopoietic stem cell transplantation. Blood. 2008;112:2120–8. doi: 10.1182/blood-2007-07-100222. [DOI] [PubMed] [Google Scholar]
- 4.Bouwman LH, Roos A, Terpstra OT, et al. Mannose binding lectin gene polymorphisms confer a major risk for severe infections after liver transplantation. Gastroenterology. 2005;129:408–14. doi: 10.1016/j.gastro.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Verschuren JJW, Roos A, Schaapherder AFM, et al. Infectious complications after simultaneous pancreas–kidney transplantation: a role for the lectin pathway of complement activation. Transplantation. 2008;85:75–80. doi: 10.1097/01.tp.0000297249.10654.f5. [DOI] [PubMed] [Google Scholar]
- 6.Schlapbach LJ, Aebi C, Otth M, et al. Serum levels of mannose-binding lectin and the risk of fever in neutropenic pediatric cancer patients. Pediatr Blood Cancer. 2007;49:11–16. doi: 10.1002/pbc.21097. [DOI] [PubMed] [Google Scholar]
- 7.Manuel O, Pascual M, Trendelenburg M, Meylan PR. Association between mannose-binding lectin deficiency and cytomegalovirus infection after kidney transplantation. Transplantation. 2007;83:359–62. doi: 10.1097/01.tp.0000251721.90688.c2. [DOI] [PubMed] [Google Scholar]
- 8.Sagedal S, Thiel S, Hansen TK, Mollnes TE, Rollag H, Hartmann A. Impact of the complement lectin pathway on cytomegalovirus disease early after kidney transplantation. Nephrol Dial Transplant. 2008;23:4054–60. doi: 10.1093/ndt/gfn355. [DOI] [PubMed] [Google Scholar]
- 9.Berger SP, Roos A, Mallat MJK, Fujita T, de Fijter JW, Daha MR. Association between mannose-binding lectin levels and graft survival in kidney transplantation. Am J Transplant. 2005;5:1361–6. doi: 10.1111/j.1600-6143.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- 10.Berger SP, Roos A, Mallat MJK, de Fijter JW, Daha MR. Pre-transplantation MBL levels predict patient and graft survival after simultaneous pancreas–kidney transplantation. Mol Immunol. 2007;44:156. doi: 10.1681/ASN.2007030262. [DOI] [PubMed] [Google Scholar]
- 11.Fiane AE, Ueland T, Simonsen S, et al. Low mannose-binding lectin and increased complement activation correlate to allograft vasculopathy, ischaemia, and rejection after human heart transplantation. Eur Heart J. 2005;26:1660–5. doi: 10.1093/eurheartj/ehi198. [DOI] [PubMed] [Google Scholar]
- 12.Fildes JE, Shaw SM, Walker AH, et al. Mannose-binding lectin deficiency offers protection from acute graft rejection after heart transplantation. J Heart Lung Transplant. 2008;27:1353–6. doi: 10.1016/j.healun.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 13.de VB, Walter SJ, Peutz-Kootstra CJ, Wolfs TG, van Heurn LW, Buurman WA. The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia-reperfusion injury. Am J Pathol. 2004;165:1677–88. doi: 10.1016/S0002-9440(10)63424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou W, Farrar CA, Abe K, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105:1363–71. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munster JM, van der Bij W, Breukink MB, et al. Association between donor MBL promoter haplotype and graft survival and the development of BOS after lung transplantation. Transplantation. 2008;86:1857–63. doi: 10.1097/TP.0b013e31819064b8. [DOI] [PubMed] [Google Scholar]
- 16.Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from Southeast Africa and South America. J Immunol. 1998;161:3169–75. [PubMed] [Google Scholar]
- 17.Garred P, Voss A, Madsen HO, Junker P. Association of mannose-binding lectin gene variation with disease severity and infections in a population-based cohort of systemic lupus erythematosus patients. Genes Immun. 2001;2:442–50. doi: 10.1038/sj.gene.6363804. [DOI] [PubMed] [Google Scholar]
- 18.Lee SG, Yum JS, Moon HM, et al. Analysis of mannose-binding lectin 2 (MBL2) genotype and the serum protein levels in the Korean population. Mol Immunol. 2005;42:969–77. doi: 10.1016/j.molimm.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Seelen MA, Roos A, Wieslander J, et al. Functional analysis of the classical, alternative, and MBL pathways of the complement system: standardization and validation of a simple ELISA. J Immunol Methods. 2005;296:187–98. doi: 10.1016/j.jim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Roos A, Bouwman LH, Munoz J, et al. Functional characterization of the lectin pathway of complement in human serum. Mol Immunol. 2003;39:655–68. doi: 10.1016/s0161-5890(02)00254-7. [DOI] [PubMed] [Google Scholar]
- 21.Ghods FJ, Solgi G, Amirzargar AA, Nikbin B, Ghods AJ. High frequency of clinically significant infections and cytomegalovirus disease in kidney transplant recipients with serum mannose-binding lectin deficiency. Iran J Kidney Dis. 2009;3:28–33. [PubMed] [Google Scholar]
- 22.Hodge S, Dean M, Hodge G, Holmes M, Reynolds PN. Decreased efferocytosis and mannose binding lectin in the airway in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2011;30:589–95. doi: 10.1016/j.healun.2011.01.710. [DOI] [PubMed] [Google Scholar]
- 23.Hodge SJ, Hodge GL, Reynolds PN, Holmes MD. Differential rates of apoptosis in bronchoalveolar lavage and blood of lung transplant patients. J Heart Lung Transplant. 2005;24:1305–14. doi: 10.1016/j.healun.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Hodge S, Matthews G, Dean MM, et al. Therapeutic role for mannose-binding lectin in cigarette smoke-induced lung inflammation? Evidence from a murine model. Am J Respir Cell Mol Biol. 2010;42:235–42. doi: 10.1165/rcmb.2008-0486OC. [DOI] [PubMed] [Google Scholar]
- 25.Worthley DL, Johnson DF, Eisen DP, et al. Donor mannose-binding lectin deficiency increases the likelihood of clinically significant infection after liver transplantation. Clin Infect Dis. 2009;48:410–17. doi: 10.1086/596313. [DOI] [PubMed] [Google Scholar]
- 26.Seyfarth J, Garred P, Madsen HO. Extra-hepatic transcription of the human mannose-binding lectin gene (mbl2) and the MBL-associated serine protease 1-3 genes. Mol Immunol. 2006;43:962–71. doi: 10.1016/j.molimm.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 27.Eisen DP. Mannose-binding lectin deficiency and respiratory tract infection. J Innate Immun. 2010;2:114–22. doi: 10.1159/000228159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamosh A. Online mendelian inheritance in man, OMIMTM website. Available at: http://www.ncbi.nlm.nih.gov/omim/ (accessed 21 September 2010.
- 29.Chong WP, To YF, Ip WK, et al. Mannose-binding lectin in chronic hepatitis B virus infection. Hepatology. 2005;42:1037–45. doi: 10.1002/hep.20891. [DOI] [PubMed] [Google Scholar]
- 30.Tan Y, Liu L, Luo P, et al. Association between mannose-binding lectin and HIV infection and progression in a Chinese population. Mol Immunol. 2009;47:632–8. doi: 10.1016/j.molimm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Seppanen M, Lokki ML, Lappalainen M, et al. Mannose-binding lectin 2 gene polymorphism in recurrent herpes simplex virus 2 infection. Hum Immunol. 2009;70:218–21. doi: 10.1016/j.humimm.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Neth OW, Bacher U, Das P, et al. Influence of mannose-binding lectin genotypes and serostatus in allo-SCT: analysis of 131 recipients and donors. Bone Marrow Transplant. 2010;45:13–19. doi: 10.1038/bmt.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170:181–7. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 34.Gottlieb J, Schulz TF, Welte T, et al. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation. 2009;87:1530–7. doi: 10.1097/TP.0b013e3181a4857d. [DOI] [PubMed] [Google Scholar]
- 35.Valentine VG, Gupta MR, Walker JE, et al. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2009;28:163–9. doi: 10.1016/j.healun.2008.11.907. [DOI] [PubMed] [Google Scholar]
- 36.Luckraz H, Sharples L, McNeil K, Wreghitt T, Wallwork J. Cytomegalovirus antibody status of donor/recipient does not influence the incidence of bronchiolitis obliterans syndrome in lung transplantation. J Heart Lung Transplant. 2003;22:287–91. doi: 10.1016/s1053-2498(02)00471-0. [DOI] [PubMed] [Google Scholar]
- 37.nziger-Isakov LA, DelaMorena M, Hayashi RJ, et al. Cytomegalovirus viremia associated with death or retransplantation in pediatric lung-transplant recipients. Transplantation. 2003;75:1538–43. doi: 10.1097/01.TP.0000061607.07985.BD. [DOI] [PubMed] [Google Scholar]
- 38.Tamm M, Aboyoun CL, Chhajed PN, Rainer S, Malouf MA, Glanville AR. Treated cytomegalovirus pneumonia is not associated with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2004;170:1120–3. doi: 10.1164/rccm.200310-1405OC. [DOI] [PubMed] [Google Scholar]
- 39.Verleden GM, Dupont LJ. Azithromycin therapy for patients with bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2004;77:1465–7. doi: 10.1097/01.tp.0000122412.80864.43. [DOI] [PubMed] [Google Scholar]
- 40.Yates B, Murphy DM, Forrest IA, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2005;172:772–5. doi: 10.1164/rccm.200411-1537OC. [DOI] [PubMed] [Google Scholar]
- 41.Vanaudenaerde BM, Meyts I, Vos R, et al. A dichotomy in bronchiolitis obliterans syndrome after lung transplantation revealed by azithromycin therapy. Eur Respir J. 2008;32:832–43. doi: 10.1183/09031936.00134307. [DOI] [PubMed] [Google Scholar]


