Figure 3.
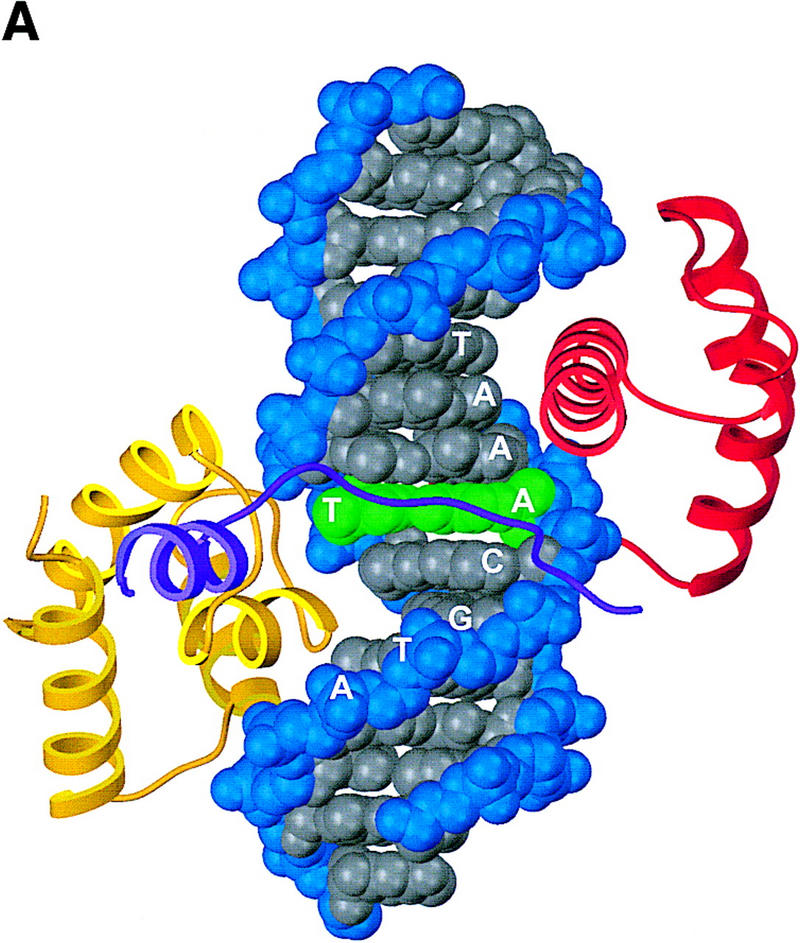
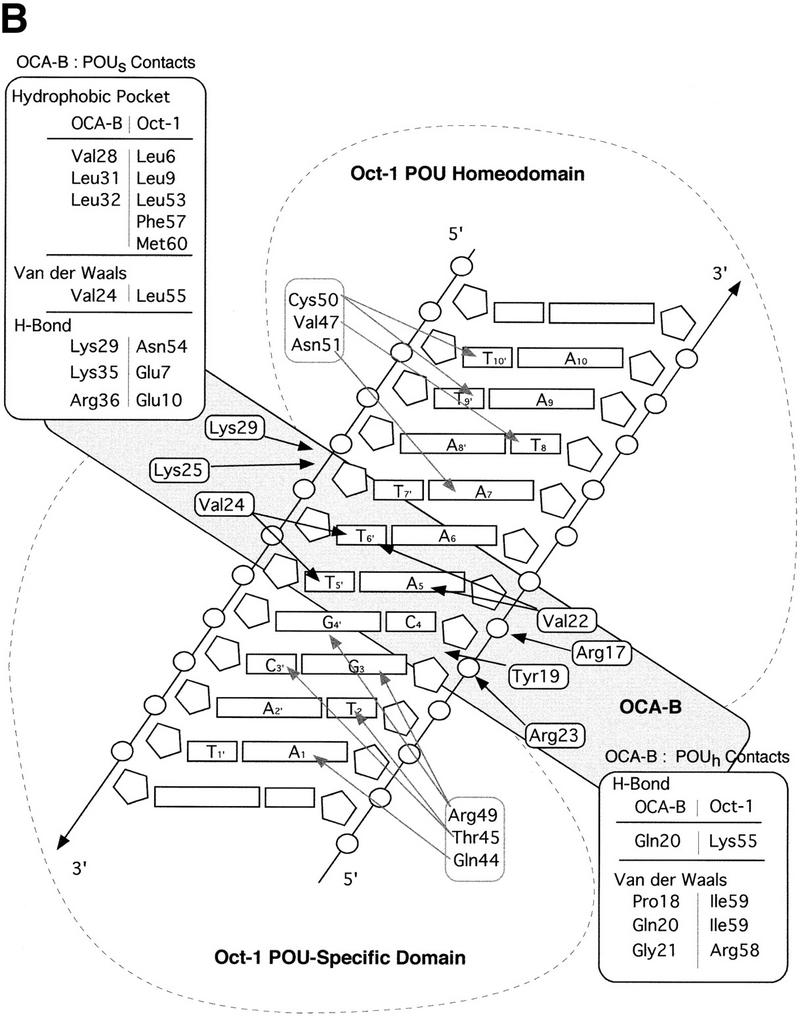
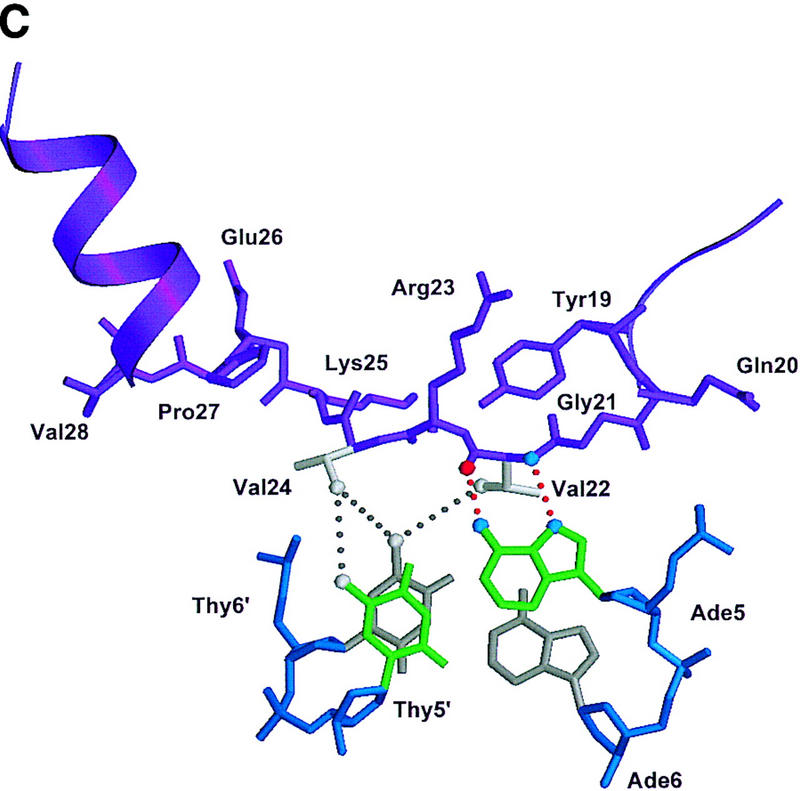
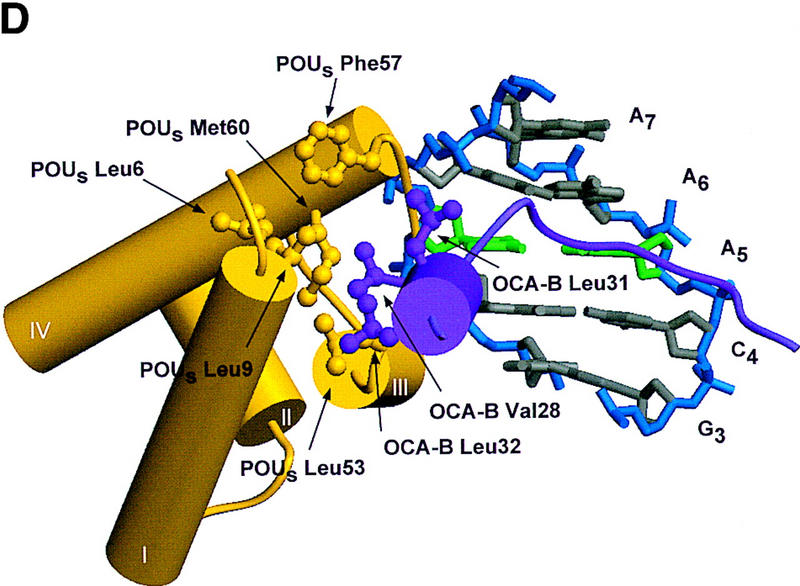
Structure of the ternary complex of the OCA-B 1–44 peptide, the Oct-1 POU domain, and an octamer element. (A) Overview of the ternary complex showing the OCA-B peptide (purple), the Oct-1 POU domain (the POU homeodomain is in red; the POU-specific domain is in yellow), and DNA containing the octamer sequence (position 5 is in green). About 20 amino acids of the OCA-B 1–44 peptide are well-ordered in the crystal. The OCA-B peptide traverses the major groove at the A:T base pair at position 5 of the octamer site. At the amino terminus of the ordered region, the OCA-B peptide makes contacts with the POU homeodomain; at the carboxyl terminus of the order region the OCA-B peptide forms a short α-helix and makes extensive contacts with the POU-specific domain. (B) Schematic summary of protein–DNA and protein–protein contacts in the ternary complex. The OCA-B 1–44 peptide is represented by the shaded rectangle across the center of the DNA; the positions of the POU-specific domain and the POU homeodomain are suggested by dashed outlines. Close contacts (<4.0 Å) between the OCA-B peptide and the POU-specific domain are summarized in the box in the upper left; close contacts with the POU homeodomain are summarized in the box in the lower right. All major DNA contacts are shown for the OCA-B peptide, but only key base contacts between the Oct-1 POU domain and the DNA are shown. The complete set of DNA contacts for the Oct-1 POU domain is very similar to those observed previously in its structure with an octamer element only (Klemm et al. 1994). (C) Contacts between the OCA-B 1–44 peptide (purple) and bases 5 (green) and 6 (gray) of the octamer element. The POU-specific domain, the POU homeodomain, and other bases in the octamer are not shown. Hydrogen bond contacts between the polypeptide backbone of the OCA-B peptide residue Val-22 and the exocyclic amine and N7 of adenine at position 5 are shown with red dots [the relevant atoms at these positions are blue (N), red (O), and gray (C)]. Hydrophobic interactions between OCA-B Val-22 and Thy-6′, and between OCA-B Val-24 and both Thy-5′ and Thy-6′ are indicated with gray dots. Residues at the amino terminus (Ala-16–Pro-18, extended chain) and at the carboxyl terminus (Lys-29-His-38, helix) of the ordered part of the OCA-B peptide are represented according to their secondary structure. (D) Interaction between the hydrophobic surface of the OCA-B peptide helix and the hydrophobic pocket of the Oct-1 POU-specific domain. POU-specific domain (yellow) residues at the amino terminus of helices one (Leu-6, Leu-9) and four (Phe-57, Met-60), and at the carboxyl terminus of helix three (Leu-53) form a shallow hydrophobic pocket. The surface of the helix from the OCA-B peptide (purple; Val-28, Leu-31, Leu-32) docks against the hydrophobic pocket of the Oct-1 POU-specific domain. The helices of the POU-specific domain are labeled with Roman numerals. Bases for one strand of the octamer element are also indicated.
