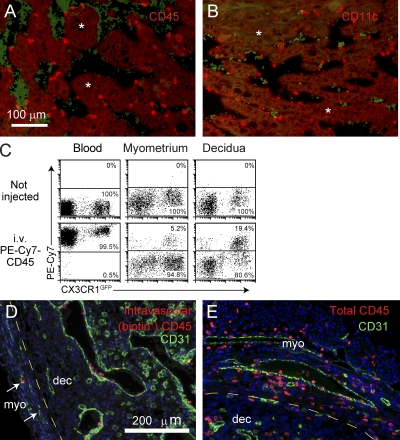
Figure 2.
Technique for assessing leukocyte blood/tissue partitioning. (A and B) Leukocyte localization in the decidual vascular zone. Tissue sections from E8.5 implantation sites were stained with anti-CD45 (A) or anti-CD11c (B) Abs (red). Vascular sinusoids are the dark areas containing scattered red blood cells that are green-tinged as a byproduct of the staining protocol. Some areas of the decidual parenchyma are indicated with asterisks. Data are representative of at least n = 6 mice from at least six independent experiments. (C) Visualization of leukocyte blood/tissue partitioning in the blood and the E8.5 myometrium and decidua. Pregnant CX3CR1GFP mice were i.v. injected with PE-Cy7–conjugated anti-CD45 Abs 1 min before sacrifice. Total leukocytes in blood and tissue cell preparations were subsequently identified with Pacific orange–conjugated anti-CD45 Abs. Intravascular leukocytes appear as PE-Cy7+, whereas extravascular cells appear as PE-Cy7−. Data are representative of n = 6 mice from four independent experiments. (D and E) Histological validation. Tissue sections from E8.5 pregnant mice injected with biotin-conjugated anti-CD45 Abs 5 min before sacrifice were double stained with anti-CD31 Abs (green) and either streptavidin (D; red) or anti-CD45 Abs (E; red). The areas shown are at the border between the myometrium (myo) and mesometrial decidua (dec), where leukocytes are present in the parenchyma of both tissue layers. Note that all biotin+ cells are present within CD31+ vessels, even within the myometrium (left; arrows). Nonvascular CD31+ cells are decidual NK cells. Data are representative of n = 2 mice from two independent experiments. DAPI was used as a counterstain.