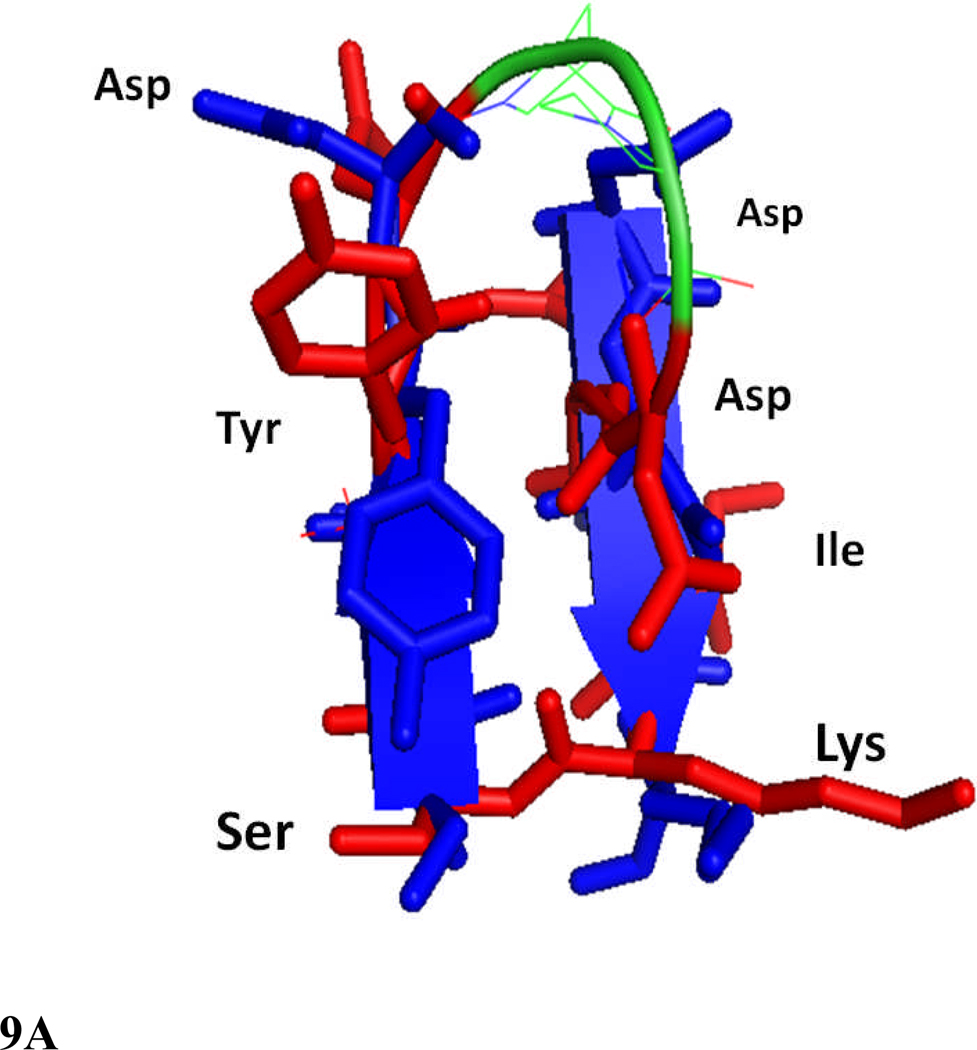
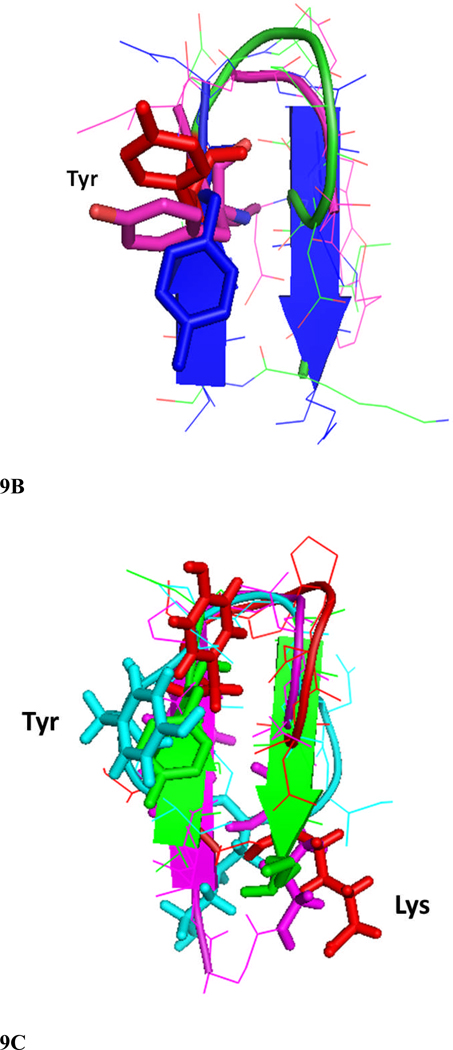
Figure 9.
Comparison of structures of peptides 6 and 7 with the F and C strands of crystal structure of CD2 protein. A) Peptide 6 backbone overlapped with F and C strands of CD2 structure. CD2 is shown in blue, peptide 6 backbone is shown in green and residues are shown as red sticks. Notice the overlap of similar residues in the crystal structure and in peptide 6. B) Overlap of backbone atoms of CD2 F and C strands with peptides 6 and 7. F and C strands of CD2, blue; peptide 6, red; peptide 7, magenta. Notice that the tyrosine residue from the hot-spot region of CD2 protein overlaps with the Tyr residue in the β-hairpin peptides 6 and 7. C) Overlapping of peptide 6 (red) structure with F and C strands of CD2 protein (green), and peptides 2 (magenta) and 3 (cyan) from our previous report. Notice that Tyr of peptide 2 has a different orientation than peptide 6 and F and C strands in crystal structure of CD2, and the Lys side chain is cyclized in peptide 2 by side chain cyclization strategy. Peptide 3 has Lys and Tyr (cyan) in different orientation compared to peptide 6 and CD2 F and C strands. Sequence of peptide 2, Cyclo(1,11) H-E1S2I3Y4D5P6G7D8D9I10K11-OH (side chain cyclization); and 3, Cyclo(1,10) E1I2Y3D4P5G6D7D8I9K.10