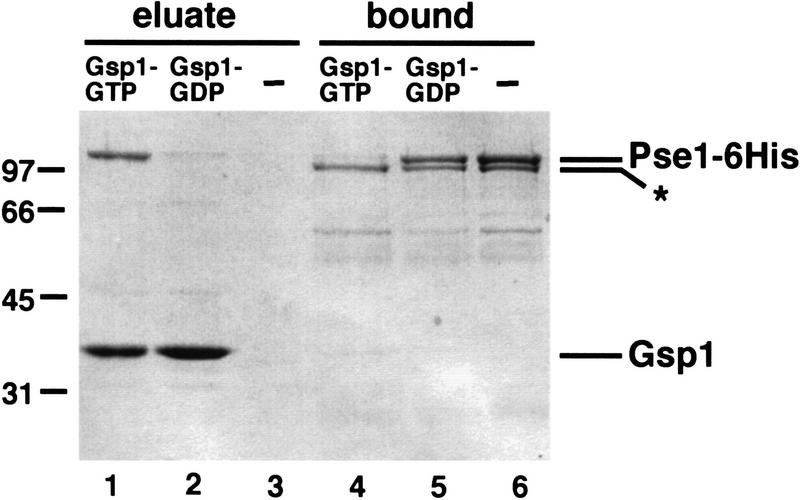
Figure 6.
Gsp1–GTP dissociates the Pho4–Pse1 complex. Purified Pse1–6His and Pho4WT–zz were incubated and the Pse1–Pho4WT–zz complex was purified with IgG–Sepharose beads. The resin was washed extensively, split into three equal parts, and incubated with Gsp1–GTP, Gsp1–GDP, or buffer alone. Proteins released during this treatment were collected as the eluate. The Pho4WT–zz resin was washed once with IgG buffer, and proteins bound to Pho4WT–zz were eluted with 1 m MgCl2 and precipitated (bound fraction). Proteins were separated on 10% SDS-PAGE and visualized by staining with Coomassie blue. Lanes 1,4) Gsp1–GTP; (lanes 2,5) Gsp1–GDP; (lanes 3,6) buffer alone. One-third of the eluate and one-half of the bound proteins were loaded onto the gel. The amino-terminally truncated form of Pse1–6His (*), which cannot bind Ran–GTP (data not shown), is not dissociated from Pho4WT–zz by incubation with Gsp1–GTP.