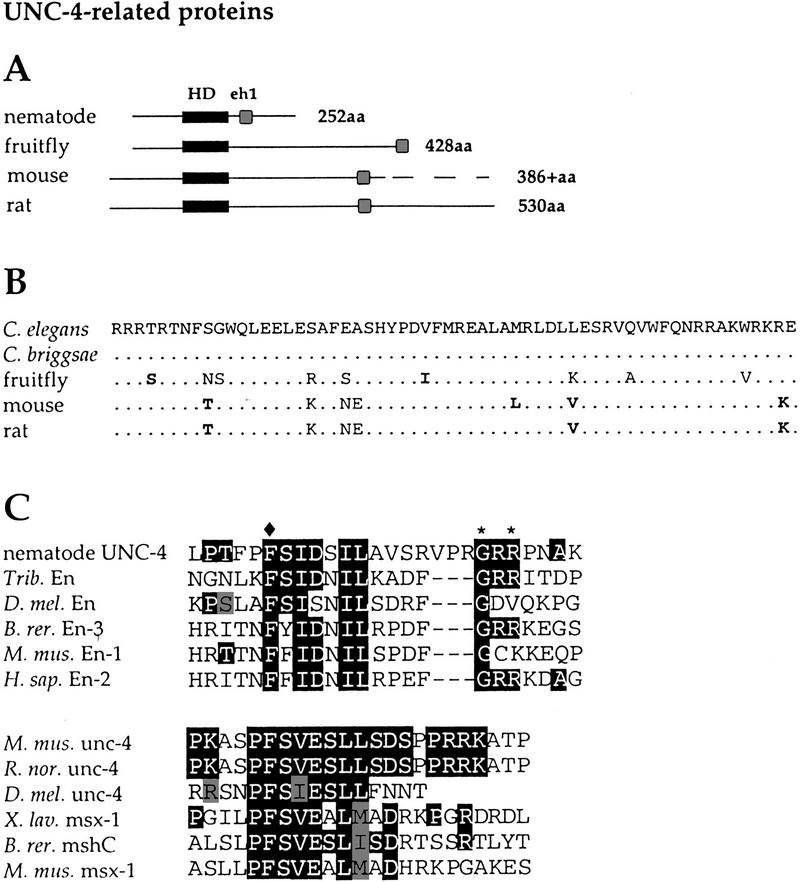
Figure 2.
Comparison of UNC-4-related proteins. (A) Schematic of UNC-4-related proteins showing relative positions of the homeodomains (solid rectangles) and eh1 domains (shaded boxes). A complete sequence of the mouse UNC-4 protein has not been reported. (B) Homeodomain sequences of UNC-4-related proteins. Dots indicate identity to C. elegans UNC-4 sequence. Bold residues show similarity to UNC-4. (C) eh1 domains of nematode UNC-4 proteins (C. elegans and C. briggsae) are most similar to eh1 domains of Engrailed family members. Vertebrate and Drosophila UNC-4 eh1 domains most closely resemble the msh homeoprotein class of eh1 sequences. For nematode UNC-4 eh1 comparison, solid boxes indicate identity to nematode UNC-4 and shaded boxes show similarity. (♦) The position of the invariant phenylalanine; (*) residues mutated in the unc-4 alleles e26, e2307, and e2323. For the vertebrate and Drosophila unc-4 eh1 alignments, the solid boxes indicate identity between the mouse and rat UNC-4 proteins and at least one other protein in the comparison; the shaded boxes show similarity.