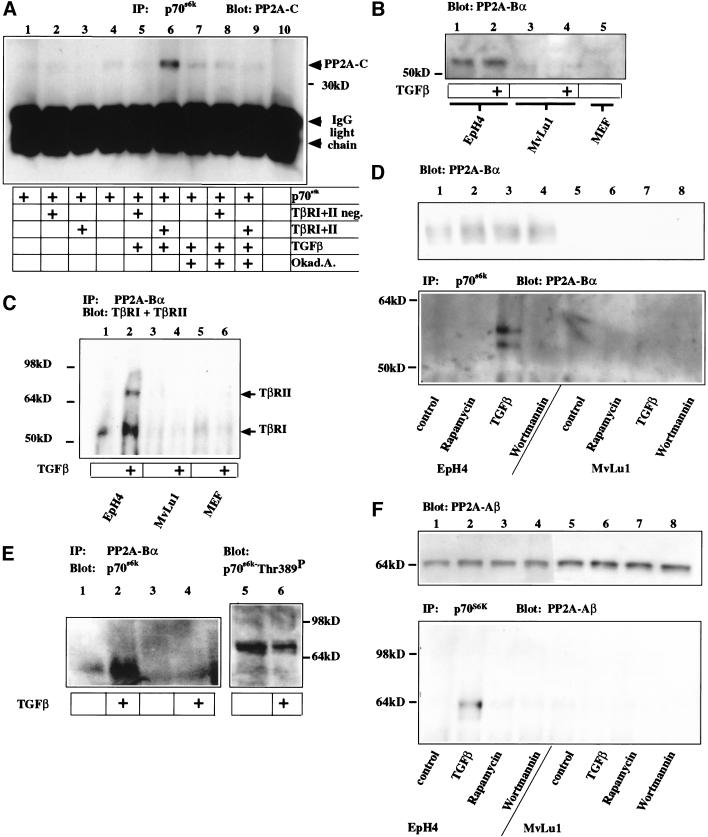
Figure 4.
TGF-β induces de-phosphorylation of p70s6k by stimulating association of PP2A subunits C, Aβ and Bα and p70s6k and association of PP2A-Bα and TβRI. (A) Western blot for PP2A-C after immunoprecipitation of p70s6k TβR expressing cells were treated with TGF-β and/or the PP2A inhibitor okadaic acid or left untreated. Interaction of PP2A with p70s6k is stimulated in cells expressing the TβRI/TβRII receptor complex on addition of TGF-β (lane 6), but inhibited by the presence of okadaic acid (Okad.A., lane 9). Coexpression of inactive TβRI/TβRII neg. fails to stimulate interaction (lanes 2,5,8) above basal binding activity (lanes 1,4,7). (B) Western blot for the expression of the endogenous regulatory subunit PP2A-Bα EpH4 epithelial cells express high levels of PP2A-Bα, and its expression is not altered by 1 h of TGF-β induction. Endogenous levels of PP2A-Bα in Mv1Lu cells and primary embryonic fibroblasts are below detection limits. (C) Immunoprecipitation for PP2A-Bα and Western blot for TβRI and TβRII. PP2A-Bα interacts with TβRI and TβRII on TGF-β stimulation in EpH4, but similar complexes are not detectable in Mv1Lu cells or primary embryonic mouse fibroblasts (MEF). (D) Western blot for PP2A-Bα after immunoprecipitation of p70s6k in EpH4 or Mv1Lu cells. PP2A-Bα interaction with p70s6k is observed specifically in response to TGF-β in EpH4 cells and not in response to other inhibitors of p70s6k or in Mv1Lu cells. (E) Western blot for p70s6k after immunoprecipitation of PP2A-Bα (lanes 1,2) or using a control antibody (lanes 3,4) and direct Western blot for Thr389 of p70s6k from cells stimulated with TGF-β in early G1. In response to TGF-β, PP2A-Bα is found in complexes with p70s6k and Thr 389 phosphorylation of p70s6k decreases. (F) Western blot for PP2A-Aβ before (top panel) and after (bottom panel) immunoprecipitation with p70s6k. In EpH4 cells, Aβ interacts with p70s6k specifically in response to TGF-β.