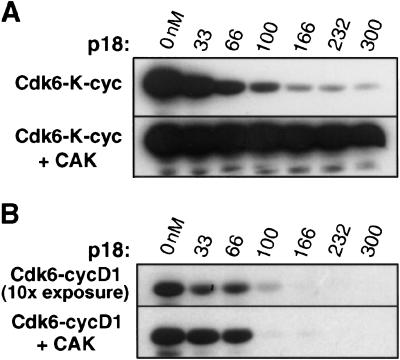
Figure 1.
The Rb kinase activity of the Cdk6–K-cyclin complex is inhibited by p18 when Cdk6 is unphosphorylated but not when Cdk6 is phosphorylated by the Cdk7–cyclinH CDK-activating kinase. (A) Phosphorylation of the Rb C-terminal fragment by the unphosphorylated Cdk6–K-cyclin complex (top panel) and the phosphorylated Cdk6–K-cyclin complex (lower panel). Both Cdk6 complexes are at 100 nM concentration. The lanes in the two panels contain p18 at 0, 33, 66, 100, 166, 232, and 300 nM concentrations. (B) Comparison with the Rb kinase activity of the Cdk6–cyclinD complex. Reactions contain the same concentration (100 nM) of Cdk6 and p18 as in the corresponding lanes in A. The gel in the bottom panel was exposed for the same length of time as the panels in A; the top panel showing the activity of unphosphorylated Cdk6–cyclinD complex was exposed 10-fold longer because of the low kinase activity of the unphosphorylated complex.