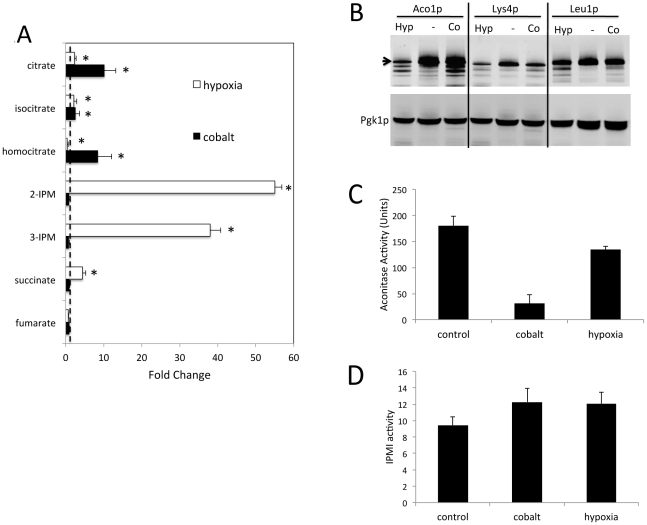
Figure 7. Effects of cobalt on Fe-S enzymes.
Cells were grown in minimal medium as in Fig. 1 and were assayed for (A) designated metabolites by GC/MS; (B,C) protein levels and enzymatic activity of indicated Fe-S proteins. A) Levels of select Fe-S enzyme substrates and products presented as fold change over control untreated samples as in Fig. 1B,C. Statistically significant differences over control (P value ≤0.05) are designated with asterisks. Dotted line = 1.0 value assigned to control. Citrate and isocitrate - substrate and product of Aconitase; homocitrate - homoaconitase substrate; 2-IPM and 3-IPM (isopropyl malate) - substrate and product of IPMI (Leu1p); succinate and fumarate - substrate and product of succinate dehydrogenase. B) Immunoblot analysis of cells expressing TAP tagged versions of Aco1p (aconitase 106 kDa), Lys4p (homoaconitase 96 kDa) and Leu1p (IPMI 106.5 kDa). Results are representative of three (Leu1p), four (Lys4p) and five (Aco1p) experimental trials. C–D) Cell lysates prepared and assayed for aconitase (C) and IPMI (D) activity as described in Materials and Methods. 1 unit of activity is defined as 1 nmole of substrate consumed per min per mg of protein.