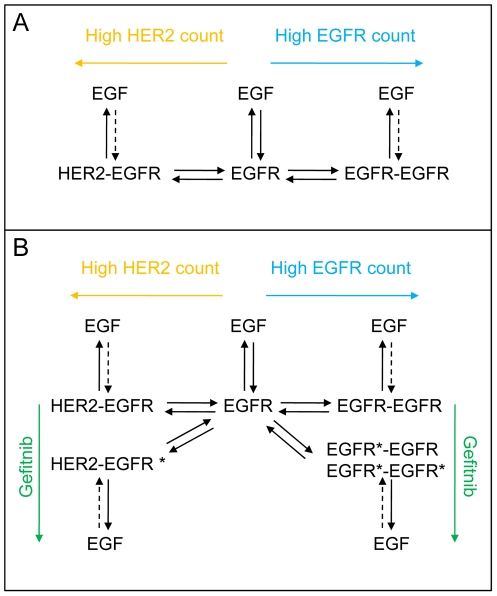
Figure 6. Proposed mechanism of EGF binding to EGFR in normal conditions and when treated with gefitinib.
A) Real-time binding data of 125I-EGF to A431 and SKOV3 cells, as presented in Interaction Maps, depicts a reality where EGF interacts with not only one receptor form, but several. The different receptor populations may be the monomeric, heterodimeric and homodimeric form of EGFR. The equilibration between the different monomer/dimer states will likely be dependent on the number of EGFR and HER2 receptors expressed on the cell surfaces, where a large HER2 count shifts the equilibrium to more EGFR–HER2 heterodimers and a high EGFR expression results in more EGFR homodimers. Differences in stability of the interaction imply that EGF can dissociate from all three forms. The ability of EGF to bind to ligand free dimers (dashed lines) remains unclear, as the existence of such dimers. B) When treated with gefitinib, the binding of EGF to the dimeric form is altered, suggesting that a new dimeric form is created, either as an addition (A431) or as a replacement (SKOV3).