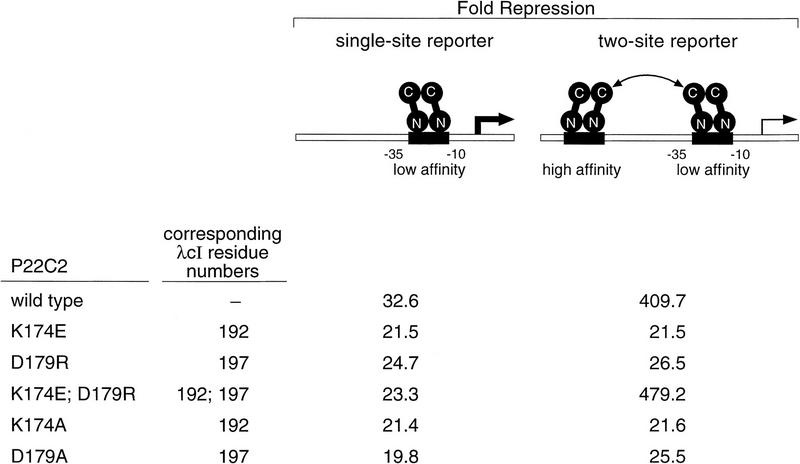
Figure 7.
Cooperative binding of wild-type and mutant variants of P22c2. Strains DV59 (single-site reporter) and DV72 (two-site reporter) were transformed with wild-type or mutant versions of plasmid pPR4 encoding P22c2. Fold repression was determined by calculation of the ratio of β-galactosidase activity observed in each case to that observed in cells transformed with the control plasmid that lacks the P22c2 gene (4404 and 4231 Miller units in strains DV59 and DV72, respectively). All cultures were grown in medium containing 10 μm IPTG. Results are averages of duplicate assays that varied by less than ±3% from the mean. Repression levels are higher than those of Figure 4A because pPR4 (which resembles the plasmid used in the initial characterization of this system; Valenzuela and Ptashne 1989) directs expression of high levels of P22c2. Note that all of the mutant proteins repressed transcription somewhat less efficiently than the wild-type protein in the single-site reporter strain, reflecting a corresponding decrease in the fractional occupancy of the P22 operator on this reporter template. The double mutant, however, repressed transcription slightly more efficiently than the wild-type protein in the two-site reporter strain (a difference that was reproducible).Therefore, we conclude that the double mutant dimers interact at least as efficiently as the wild-type dimers.