Figure 2.
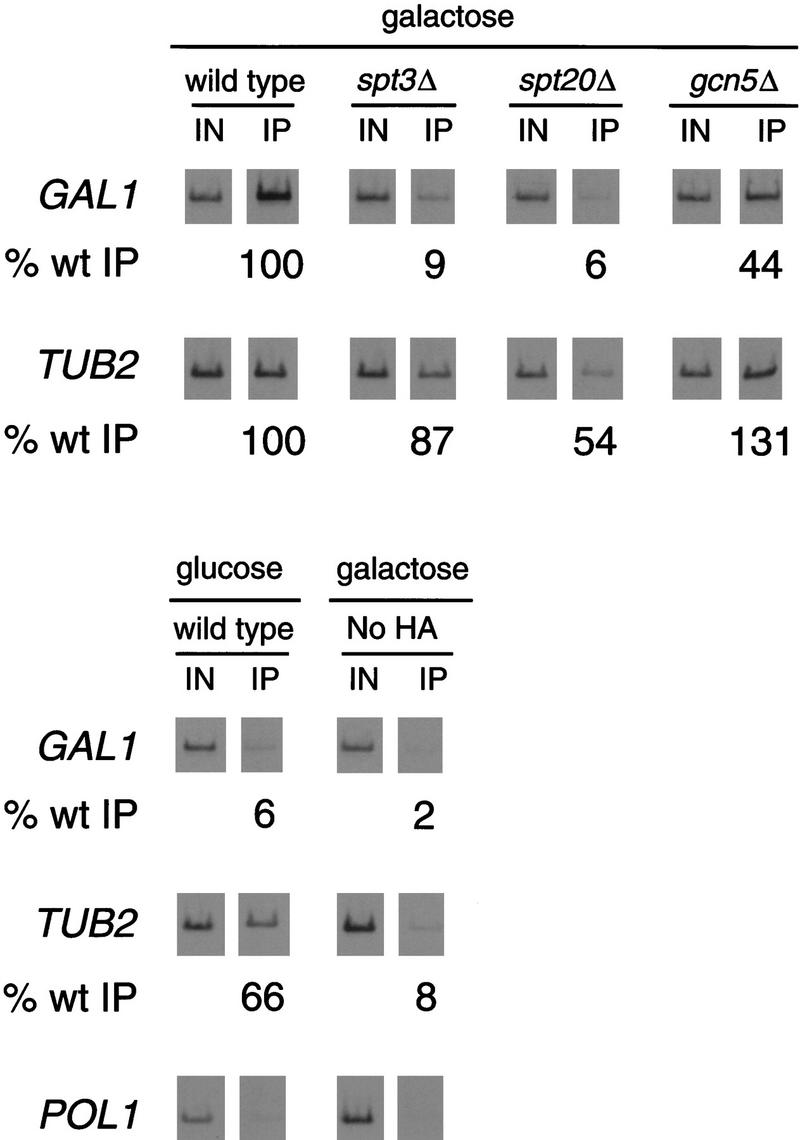
TBP is not bound to the GAL1 TATA in spt3Δ and spt20Δ mutants. Chromatin immunoprecipitation was performed in parallel with RNA analysis (Fig. 1) from glucose-repressed wild-type strains and galactose-induced wild-type, spt3Δ, spt20Δ, and gcn5Δ strains. A galactose-induced wild-type strain that contained TBP without the triple HA tag (no HA) was included as a negative control for the immunoprecipitation. TBP was immunoprecipitated using the 12CA5 antibody against the HA epitope. PCR products correspond to the GAL1 TATA region (GAL1), the TUB2 TATA region (TUB2), or the POL1 open reading frame as a negative control. The percentage of DNA immunoprecipitated (% wt IP) in each of the mutants was normalized to the amount immunoprecipitated from the galactose-induced wild-type strain. One set of PCR reactions is shown, and the quantitation represents the average of several experiments. The average values with standard errors for the measurements of TBP binding to the GAL1 TATA on galactose-grown cells are as follows: spt3Δ, 9 ± 2; spt20Δ, 6 ± 2; gcn5Δ, 44 ± 3; No HA, 2 ± 1. The values for binding to the TUB2 TATA on galactose-grown cells are: spt3Δ, 87 ± 34; spt20Δ, 54 ± 10; gcn5Δ, 131 ± 22; No HA, 8 ± 2. The values for wild-type glucose-grown cells are as follows: GAL1, 6 ± 3; TUB2, 66 ± 8. The low level of DNA detected in the No-HA and the POL1 PCR reactions represents the low amount of TBP-independent DNA precipitated as background in this assay. The POL1 negative control was performed on all samples, and the results were essentially the same as the example shown.
